��Ŀ����
����Ŀ���Ե���Ϊ��Ҫԭ�Ϻϳ�һ���й���ζ������C�ͻ�����D�ĺϳ�·������ͼ��ʾ��
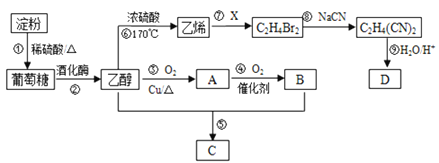
��֪��![]()
��ش��������⣺
��1��A�Ľṹ��ʽΪ____________________��B�����еĹ���������Ϊ__________________��
��2����Ӧ�ߵĻ�ѧ����ʽΪ___________________________����Ӧ�������Ϊ______________��
��3����Ӧ�ݵĻ�ѧ����ʽΪ_____________________________��
��4����֪D����Է�����Ϊ118����������ֻ����һ�ֹ����ţ�����̼������Ԫ�ص����������ֱ�Ϊ40.68%��5.08%������Ϊ��Ԫ�أ���D�Ľṹ��ʽΪ________________________________��
���𰸡�CH3CHO�Ȼ�CH2=CH2 +Br2 �� CH2BrCH2Brȡ����ӦCH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2OHOOC-CH2-CH2-COOH
CH3COOCH2CH3��H2OHOOC-CH2-CH2-COOH
��������
��ϩ�Ľṹ��ʽΪCH2=CH2����ϩ��X��Ӧ����C2H4Br2����XΪBr2��C2H4Br2�Ľṹ��ʽΪBrCH2CH2Br��BrCH2CH2Br��NaCN��Ӧ����C2H4��CN��2���÷�ӦΪȡ����Ӧ��C2H4��CN��2�Ľṹ��ʽΪNCCH2CH2CN��CH3CH2OH��Cu�����·�����������Ӧ����CH3CHO��CH3CHO����������Ӧ����CH3COOH��CH3CH2OH��CH3COOH����������Ӧ���ɾ��й���ζ��CH3COOCH2CH3��A��B��C�Ľṹ��ʽ����ΪCH3CHO��CH3COOH��CH3COOCH2CH3�����������ƶ�����
��ϩ�Ľṹ��ʽΪCH2=CH2����ϩ��X��Ӧ����C2H4Br2����XΪBr2��C2H4Br2�Ľṹ��ʽΪBrCH2CH2Br��BrCH2CH2Br��NaCN��Ӧ����C2H4��CN��2���÷�ӦΪȡ����Ӧ��C2H4��CN��2�Ľṹ��ʽΪNCCH2CH2CN��CH3CH2OH��Cu�����·�����������Ӧ����CH3CHO��CH3CHO����������Ӧ����CH3COOH��CH3CH2OH��CH3COOH����������Ӧ���ɾ��й���ζ��CH3COOCH2CH3��A��B��C�Ľṹ��ʽ����ΪCH3CHO��CH3COOH��CH3COOCH2CH3��
��1����������������A�Ľṹ��ʽΪCH3CHO��B�Ľṹ��ʽΪCH3COOH��B�к��еĹ���������Ϊ�Ȼ���
��2����Ӧ��Ϊ��ϩ��Br2�ļӳɷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪCH2=CH2+Br2��BrCH2CH2Br���Ա�C2H4Br2��C2H4��CN��2���ɼ���Ӧ��ΪC2H4Br2��NaCN��ȡ����Ӧ��
��3����Ӧ��ΪCH3CH2OH��CH3COOH��������Ӧ����Ӧ�Ļ�ѧ����ʽΪCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��4��1��D���Ӻ�̼ԭ����N��C��=![]() =4����1��D�����к���ԭ����N��H��=
=4����1��D�����к���ԭ����N��H��=![]() =6����1��D�����к���ԭ����N��O��=��118-4��12-6��1����16=4����D�ķ���ʽΪC4H6O4��D��������ֻ����һ�ֹ����ţ�C2H4��CN��2�Ľṹ��ʽΪNCCH2CH2CN����Ӧ�����������֪�ķ�Ӧ��D�Ľṹ��ʽΪHOOCCH2CH2COOH��
=6����1��D�����к���ԭ����N��O��=��118-4��12-6��1����16=4����D�ķ���ʽΪC4H6O4��D��������ֻ����һ�ֹ����ţ�C2H4��CN��2�Ľṹ��ʽΪNCCH2CH2CN����Ӧ�����������֪�ķ�Ӧ��D�Ľṹ��ʽΪHOOCCH2CH2COOH��

 �������ϵ�д�
�������ϵ�д�����Ŀ�����й������ʷ������ȷ����� ( )
�� | �� | �� | ���������� | ���������� | |
A | Na2CO3 | H2SO4 | (NH4)2CO3 | MgO | CO2 |
B | NaOH | HCl | NaCl | Na2O | H2O |
C | Ba(OH)2 | H2CO3 | CaCl2 | CO2 | SO2 |
D | KOH | HNO3 | CaCO3 | CaO | SO3 |
A. A B. B C. C D. D
����Ŀ������ڷ��ٷ�չ����������Ҳ���ܹ�ע����μ�����Ⱦ��Ѱ�������Դ���ִ�ѧ���о��ķ���
����Ϊ��ģ������β���ڴ�ת�����ڵĹ������
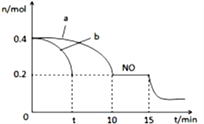
��1�������������÷�Ӧ�ں����ܱ������н������·�Ӧ:2NO(g)+2CO(g) ![]() N2(g)+2CO2(g)���ô�������ò�ͬʱ��NO��CO��Ũ�����±���
N2(g)+2CO2(g)���ô�������ò�ͬʱ��NO��CO��Ũ�����±���
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
c(NO)(10-4mol/L) | 10.0 | 4.50 | 2.50 | 1.50 | 1.00 | 1.00 |
c(CO)(10-3mol/L) | 3.60 | 3.05 | 2.85 | 2.75 | 2.70 | 2.70 |
ǰ2s�ڵ�ƽ����Ӧ����v(N2)=_______
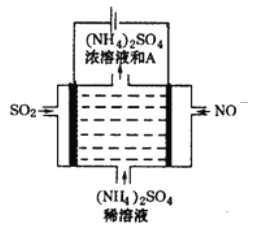
��2���ð�����������ԭ������(NOx)
�ٸ�����ͼ (������������Ч�ʡ�������Ϊ�������ʵ���֮��)���ж��������Ч�ʵ���������ǣ�___��

����֪: N2(g)+O2(g)=2NO(g)��H=+akJ/mol
N2(g)+3H2(g) ![]() 2NH3(g)��H=-bk/mol
2NH3(g)��H=-bk/mol
2H2(g)+O2(g)=2H2O(g)��H=-ckJ/mol����a��b��c������0��
����ȷ�Ӧ:4NO(g)+4NH3(g)+O2(g) ![]() 4N2(g)+6H2O(g)��H=________��
4N2(g)+6H2O(g)��H=________��
�����״���һ�ֺܺõ�ȼ��
��1����ѹǿΪ0.1MPa�����£�a mol CO��3a mol H2�Ļ�������ڴ������������Է���Ӧ���ɼ״���CO��g��+2H2��g��CH3OH��l�� ��H��0 ��Ϊ��Ѱ�Һϳɼ״��������¶Ⱥ�ѹǿ��ijͬѧ���������ʵ�飬����ʵ�������Ѿ������������ʵ����Ʊ��У�
ʵ���� | T������ | n��CO��/n��H2�� | P��MPa�� |
�� | 180 | 2��3 | 0.1 |
�� | n | 2��3 | 5 |
�� | 350 | m | 5 |
����ʣ���ʵ�����ݣ�n=________��m=________��
��2��CH3OHȼ�ϵ����Ŀǰ������ɹ���ȼ�ϵ��֮һ������ȼ�ϵ���ɼ״�������(����)��KOH(�������Һ)���ɡ�����˵����ȷ����______________(�����)��
�ٵ�طŵ�ʱͨ������ĵ缫Ϊ����
�ڵ�طŵ�ʱ���������Һ�ļ�������
�۵�طŵ�ʱÿ����6.4 g CH3OHת��1.2 mol����
�ܸ�����ӦʽΪCH3OH��8OH����6e��=CO32-+6H2O