��Ŀ����
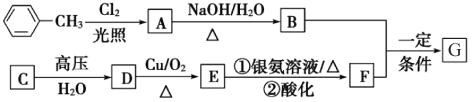
����Ŀ����֪A��B����������Ԫ����ɵĻ����A��ij��Ԫ�ص���������Ϊ![]() ��B��һ�ֵ���ɫ���壬C��J��ͬ����Ԫ�ص���̬�⻯�����C�Ǻ�������ߵ�����DΪ��������ǿ����������ǿ��İ�ɫ��״���ʣ�XΪ��������ɫҺ�塣��Ӧ���ɵ�ˮ������ȥ������������ͼ��ʾ�Ĺ�ϵ��
��B��һ�ֵ���ɫ���壬C��J��ͬ����Ԫ�ص���̬�⻯�����C�Ǻ�������ߵ�����DΪ��������ǿ����������ǿ��İ�ɫ��״���ʣ�XΪ��������ɫҺ�塣��Ӧ���ɵ�ˮ������ȥ������������ͼ��ʾ�Ĺ�ϵ��
(1)д��A�Ļ�ѧʽ��________
(2)д�� B�ĵ���ʽ______________
(3)��Ӧ![]() ��ÿ����
��ÿ����![]() ��ת�Ƶ��ӵ���ĿΪ________
��ת�Ƶ��ӵ���ĿΪ________![]() ��
��![]() ��ʾ�����ӵ�����
��ʾ�����ӵ�����![]()
(4)��Ӧ![]() �Ļ�ѧ����ʽΪ��______________________________
�Ļ�ѧ����ʽΪ��______________________________
(5)д��H����ʱ��Ӧ![]() �����ӷ���ʽ_______________________________________
�����ӷ���ʽ_______________________________________
���𰸡�![]()
![]()
![]() 4NH3+5O2
4NH3+5O2![]() 4NO+6H2O
4NO+6H2O ![]()
��������
������Ŀ������Ϣ������ɫ���壬��������ߵ����Ʋ�����ʣ�������Щ���ʵ����ʣ��ƶϳ��������ʵ����ʣ���д����ѧ����ʽ�����ӷ���ʽ��
C�Ǻ�������ߵ�������Ϊ���飻DΪ��������ǿ����������ǿ��İ�ɫ��״���ʣ�Ϊ����������B�ǵ���ɫ���壬��B����X��Ӧ����E��F����BΪ![]() ��EΪNaOH��FΪ
��EΪNaOH��FΪ![]() ��XΪ
��XΪ![]() ��D(��������)����E��Ӧ����G����GΪƫ�����ƣ�C��F��Ӧ����H����HΪ������̼����̬�⻯��J��F(����
��D(��������)����E��Ӧ����G����GΪƫ�����ƣ�C��F��Ӧ����H����HΪ������̼����̬�⻯��J��F(����![]() ��Ӧ�õ�K��K��F(����)��Ӧ�õ�L��L��X(ˮ)��Ӧ�õ�I��K����ѧ�й�ҵ�Ʊ��������ת����ϵ������֪JΪ
��Ӧ�õ�K��K��F(����)��Ӧ�õ�L��L��X(ˮ)��Ӧ�õ�I��K����ѧ�й�ҵ�Ʊ��������ת����ϵ������֪JΪ![]() ��KΪNO��LΪ
��KΪNO��LΪ![]() ��IΪ
��IΪ![]() ��CΪ
��CΪ![]() ��DΪ������������A�к���C��AlԪ�أ���AΪ
��DΪ������������A�к���C��AlԪ�أ���AΪ![]() ��������Ԫ����������Ϊ
��������Ԫ����������Ϊ![]() ���������⡣
���������⡣
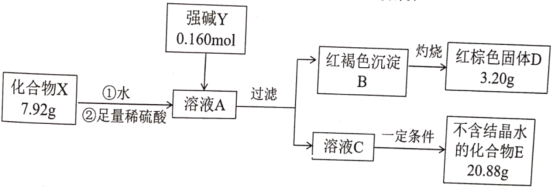
(1)������������֪��AΪ![]() ��
��
(2)![]() �ǹ������ƣ�����ʽΪ
�ǹ������ƣ�����ʽΪ![]() ��
��
(3)��Ӧ![]() ��ˮ��Ӧ�����������ƺ�������2Na2O2+2H2O=4NaOH+O2����������������Ԫ�صĻ��ϼ۴�-1���͵�-2���ĸ���ԭ�ӣ�������ԭ�Ӵ�-1���ߵ�0�ۣ�����������ԭ�Ӵ�-1�۽��͵�-2�ۣ�ÿ����
��ˮ��Ӧ�����������ƺ�������2Na2O2+2H2O=4NaOH+O2����������������Ԫ�صĻ��ϼ۴�-1���͵�-2���ĸ���ԭ�ӣ�������ԭ�Ӵ�-1���ߵ�0�ۣ�����������ԭ�Ӵ�-1�۽��͵�-2�ۣ�ÿ����![]() ��ת�Ƶ���2mol��ת�Ƶ��ӵ���ĿΪ
��ת�Ƶ���2mol��ת�Ƶ��ӵ���ĿΪ![]() ��
��
(4)��Ӧ![]() Ϊ�����Ĵ���������ѧ����ʽΪ��4NH3+5O2
Ϊ�����Ĵ���������ѧ����ʽΪ��4NH3+5O2![]() 4NO+6H2O��
4NO+6H2O��
(5)��Ӧ![]() Ϊ
Ϊ![]() ��
��![]() �ķ�Ӧ��
�ķ�Ӧ��![]() ����ʱ�����߷�Ӧ��������������̼��������ӷ���ʽΪ��
����ʱ�����߷�Ӧ��������������̼��������ӷ���ʽΪ��![]() ��
��

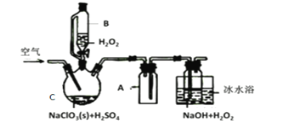
����Ŀ����ѧ���۽�Ϊ������û�ѧʵ�����������ۣ����ʹ���۾���Ȥζ�ԣ�ij�༶��ѧѧϰС��ͨ��ʵ�����Ʊ�CO2�ķ�Ӧ̽��ijЩ��ѧ���ۡ��±���ʵ������е����ݼ������Ϣ��
��� | ��Ӧ �¶�/�� | c(HCl)/(mol��L-1) | V(HCl)/mL | 10g CaCO3 ����״ | t/min |
�� | 20 | 2 | 10 | ��״ | t1 |
�� | 20 | 4 | 10 | ��״ | t2 |
�� | 20 | 2 | 10 | ��״ | t3 |
�� | 40 | 2 | 10 | ��״ | t4 |
�� | 40 | 4 | 10 | ��״ | t5 |
![]() ��ʾ�ռ�CO2���Ϊa mL�����ʱ�䡣ע���������������ͬ�����²�á�
��ʾ�ռ�CO2���Ϊa mL�����ʱ�䡣ע���������������ͬ�����²�á�
(1)�ɱ��е���Ϣ��֪��ʵ���Ŀ����̽��__________��
(2)�����е�ʵ��ٺ�ʵ�����̽��_____�Ի�ѧ��Ӧ���ʵ�Ӱ�졣���������е���Ϣ��֪��Ӱ��û�ѧ��Ӧ���ʵ����ػ���______________________________��
(3)�ռ�a mLCO2�����ʱ�����ٵ���ʵ��______________________________��
����Ŀ�������£�������ĵ���ƽ�ⳣ�����£�
��ѧʽ | HF | HCN | H2CO3 |
���볣�� | Ka=3.5��10-4 | Ka=5.0��10-10 | Ka1=4.4��10-7 Ka2=4.7��10-11 |
��1��c(H+)��ͬ����������Һ��Ũ�ȴӴ�СΪ___��
��2����HCN��Һ����ʼŨ��Ϊ0.01mol��L-1��ƽ��ʱc(H+)ԼΪ__mol��L-1��ʹ����Һ��HCN�ĵ���̶�������c(H+)Ҳ����ķ�����__��
��3���к͵�����NaOH�����ĵ�pH�����������������ֱ�ΪaL��bL����a__(������������С������������������ͬ)b���к͵�Ũ�ȡ��������������������ҪNaOH�����ʵ���Ϊn1��n2����n1__n2��
��4����NaCN��Һ��ͨ��������CO2��������Ӧ�����ӷ���ʽΪ__��
��5�����ʵ��֤��������HCl��������__��