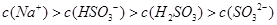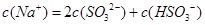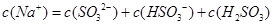��Ŀ����
ij��ҵ��ˮ�к���CN-��Cr2O �����ӣ��辭��ˮ������������ŷţ���ˮ�����������������̽��д�����
�����ӣ��辭��ˮ������������ŷţ���ˮ�����������������̽��д�����

�ش��������⣺
��1��������У�CN-��ClO-����ΪCNO-�����ӷ���ʽΪ________________��
��2������۵ķ�ӦΪS2O32����Cr2O72����H�� SO42����Cr3����H2O��δ��ƽ������ÿ����0.4mol Cr2O72��ת��__________mol e-��
SO42����Cr3����H2O��δ��ƽ������ÿ����0.4mol Cr2O72��ת��__________mol e-��
��3����Cr3+��ˮ���Լ�����ʯ�ҽ�һ��������Ŀ����____________________��
��4����25���£���amol/L��NaCN��Һ��0.01mol/L������������ϣ���Ӧ������ҺpH��7����a________0.01�����������������=�������ú�a�Ĵ���ʽ��ʾHCN�ĵ��볣��Ka��_________________��
��5��ȡ��ҵ��ˮˮ�����Թ��У�����NaOH��Һ�۲쵽����ɫ�������ɣ������������ٲ�����ɫ����Ϊֹ��������Һ�м�������Na2S��Һ����ɫ����ת���ɺ�ɫ�������ù����з�Ӧ�����ӷ�����________��
 �����ӣ��辭��ˮ������������ŷţ���ˮ�����������������̽��д�����
�����ӣ��辭��ˮ������������ŷţ���ˮ�����������������̽��д�����
�ش��������⣺
��1��������У�CN-��ClO-����ΪCNO-�����ӷ���ʽΪ________________��
��2������۵ķ�ӦΪS2O32����Cr2O72����H��
 SO42����Cr3����H2O��δ��ƽ������ÿ����0.4mol Cr2O72��ת��__________mol e-��
SO42����Cr3����H2O��δ��ƽ������ÿ����0.4mol Cr2O72��ת��__________mol e-����3����Cr3+��ˮ���Լ�����ʯ�ҽ�һ��������Ŀ����____________________��
��4����25���£���amol/L��NaCN��Һ��0.01mol/L������������ϣ���Ӧ������ҺpH��7����a________0.01�����������������=�������ú�a�Ĵ���ʽ��ʾHCN�ĵ��볣��Ka��_________________��
��5��ȡ��ҵ��ˮˮ�����Թ��У�����NaOH��Һ�۲쵽����ɫ�������ɣ������������ٲ�����ɫ����Ϊֹ��������Һ�м�������Na2S��Һ����ɫ����ת���ɺ�ɫ�������ù����з�Ӧ�����ӷ�����________��
��1��CN����ClO����CNO����Cl����2�֣� ��2�� 2.4��2�֣�
��3�����ڷ�ˮpH��ʹ��ת����Cr(OH)3������ȥ��2�֣����������𰸾����֣�
��4������2�֣� (100a��1)��10��7 mol��L��1��2�֣���λ��дҲ���֣�
��5��Cu2����2OH����Cu(OH)2����2�֣���
Cu(OH)2(s)��S2��(aq)��CuS(s)��2OH��(aq)��2�֣���ע��״̬Ҳ���֣�
��3�����ڷ�ˮpH��ʹ��ת����Cr(OH)3������ȥ��2�֣����������𰸾����֣�
��4������2�֣� (100a��1)��10��7 mol��L��1��2�֣���λ��дҲ���֣�
��5��Cu2����2OH����Cu(OH)2����2�֣���
Cu(OH)2(s)��S2��(aq)��CuS(s)��2OH��(aq)��2�֣���ע��״̬Ҳ���֣�
�����������1��������У�CN����ClO������ΪCNO������Ϊ���ڼ��Ի����У�����ClO��ֻ�ܱ���ԭΪCl������˷�Ӧ�����ӷ���ʽΪCN����ClO����CNO����Cl����
��2�����ݷ���ʽ��֪���ڷ�Ӧ��CrԪ�صĻ��ϼ۴ӣ�6�۽��͵���3�ۣ��õ�3�����ӣ�����0.4 mol Cr2O72-ת��ΪCr3��ʱת�Ƶ��ӵ����ʵ�����0.4mol����6��3����2��2.4mol��
��3����ʯ����ǿ�����ˮ�Լ��ԣ����Ժ�Cr3+��ˮ�м�����ʯ�ҽ�һ��������Ŀ���ǵ��ڷ�ˮpH��ʹ��ת����Cr(OH)3������ȥ��
��4��NaCN�����ᷴӦ����HCN���Ȼ��ƣ���Ӧ������ҺpH��7����˵��NaCN���������a��mol/L�����ݵ���غ�c(Na��)��c(H��)��c(OH��)��c(Cl��)��c(CN��)�������غ�c(Na��)��c(CN��)��c(HCN)��֪��c(HCN)��c(Cl��)��0.005mol/L��c(CN��)��0.5amol/L��0.005mol/L�����Ը��¶���HCN�ĵ��볣��Ka��
 ��
�� ��(100a��1)��10��7 mol��L��1��
��(100a��1)��10��7 mol��L��1����5����ɫ����ΪCu(OH)2��CuS(s)��Cu(OH)2(s)�����ܣ������˳�����ת�������Է�Ӧ�Ļ�ѧ����ʽΪCu2����2OH����Cu(OH)2����Cu(OH)2(s)��S2��(aq)��CuS(s)��2OH��(aq)��

��ϰ��ϵ�д�
�����Ŀ
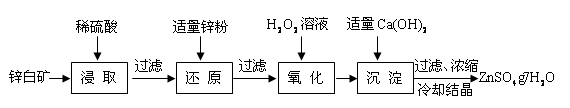

 ������Һ�У�
������Һ�У�