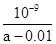��Ŀ����
����п���Ʊ�ӫ��۵�ԭ��֮һ����ҵ����п����Ҫ�ɷ���ZnO��������Fe2O3��CuO��SiO2�����ʣ��Ʊ�ZnSO4?7H2O���������¡�
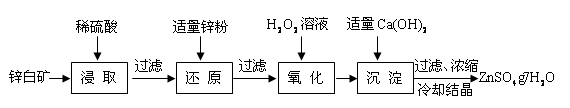
��֪�������£���Һ�е�Fe3+��Zn2+��Fe2+������������ʽ��ȫ������pH�ֱ�Ϊ��3.7��6.5��9.7��
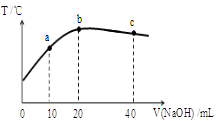
��1����ȡ��������߽���Ч�ʿɲ��õĴ�ʩ���������δ�һ������
ZnO�����ᷴӦ�Ļ�ѧ����ʽΪ������
��2����������п�۵�����Ϊ����ʹ��Һ�е�Fe3+ת��ΪFe2+����������
��3������������H2O2������Ӧ�����ӷ���ʽΪ������
��4����������Ca(OH)2������ҺpH���ٽ�Fe3+ˮ�⣬Fe3+ˮ�ⷴӦ��ƽ�ⳣ������ʽ
K��������Ca(OH)2���ܹ�����ԭ����������

��֪�������£���Һ�е�Fe3+��Zn2+��Fe2+������������ʽ��ȫ������pH�ֱ�Ϊ��3.7��6.5��9.7��
��1����ȡ��������߽���Ч�ʿɲ��õĴ�ʩ���������δ�һ������
ZnO�����ᷴӦ�Ļ�ѧ����ʽΪ������
��2����������п�۵�����Ϊ����ʹ��Һ�е�Fe3+ת��ΪFe2+����������
��3������������H2O2������Ӧ�����ӷ���ʽΪ������
��4����������Ca(OH)2������ҺpH���ٽ�Fe3+ˮ�⣬Fe3+ˮ�ⷴӦ��ƽ�ⳣ������ʽ
K��������Ca(OH)2���ܹ�����ԭ����������
��12�֣�
��1������ʯ�������߽�ȡ�¶Ȼ��ʵ��������Ũ�ȵȣ�2�֣�
ZnO+H2SO4=ZnSO4+H2O��2�֣�
��2����ȥCu2+��2�֣�
��3��H2O2+2Fe2++2H+=2Fe3++2H2O��2�֣�
��4��c3(H+)/c(Fe3+) ��2�֣� ��ֹ����Zn(OH)2��2�֣�
��1������ʯ�������߽�ȡ�¶Ȼ��ʵ��������Ũ�ȵȣ�2�֣�
ZnO+H2SO4=ZnSO4+H2O��2�֣�
��2����ȥCu2+��2�֣�
��3��H2O2+2Fe2++2H+=2Fe3++2H2O��2�֣�
��4��c3(H+)/c(Fe3+) ��2�֣� ��ֹ����Zn(OH)2��2�֣�
�����������1��������������ͬ�����������Ҫ�ɷݷ�Ӧ���ӻ�ѧ��Ӧ���ʺͻ�ѧƽ���Ӱ��������������ͨ�������¶��������п���ܽ�ȣ���ͨ������ʹ��Ӧ���ֽӴ���Ӧ����ͨ�����˺�������ν�ȡ�����ת���ʣ��ʴ�Ϊ���ʵ���߷�Ӧ�¶ȡ����ӽ���ʱ�䣨�����������𰸣���ZnO�����ᷴӦ�Ļ�ѧ����ʽΪZnO+H2SO4=ZnSO4+H2O���ʴ�Ϊ�� ZnO+H2SO4=ZnSO4+H2O���Ƽ���п�ۣ��������·�Ӧ��Zn��2H��=Zn2����H2����Zn��2Fe3��=2Fe2����Zn2�� ,Fe��Cu2�� =Cu��Zn2�� ,Zn������Fe2����Zn=Fe��Zn2�� ��������Ŀ�м���������п�ۣ��ڳ�ȥCu2�� ���𰸣���ȥCu2+����H2O2��Fe2�� ������Fe3�� ������pH�ϵ͵������£�����ˮ���ȥFe3�� ���𰸣�H2O2+2Fe2++2H+=2Fe3++2H2O���ȸ���ˮ�ⷽ��ʽFe3����3H2O
 Fe(OH)3��3H�� ,д��ƽ�ⳣ��K= c3(H+)/c(Fe3+) ��Ca(OH)2���ܹ�����ԭ���ǣ�Fe3�� ��pHΪ1.1�D3.2ʱ������pH��5.9�D8.9ʱZn2�� �������𰸣�c3(H+)/c(Fe3+)����ֹ����Zn(OH)2��
Fe(OH)3��3H�� ,д��ƽ�ⳣ��K= c3(H+)/c(Fe3+) ��Ca(OH)2���ܹ�����ԭ���ǣ�Fe3�� ��pHΪ1.1�D3.2ʱ������pH��5.9�D8.9ʱZn2�� �������𰸣�c3(H+)/c(Fe3+)����ֹ����Zn(OH)2��
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
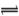 X2��+H+���ش��������⣺
X2��+H+���ش��������⣺ ��2����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��FeCl3��AlCl3�����Һ����μ��백ˮ�������� ���ѧʽ����������֪25��ʱKsp[Fe(OH)3]=2.6��10��39 mol4��L��4��KsP[Al(OH)3]=1.3��10��33 mol4��L��4��
��2����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��FeCl3��AlCl3�����Һ����μ��백ˮ�������� ���ѧʽ����������֪25��ʱKsp[Fe(OH)3]=2.6��10��39 mol4��L��4��KsP[Al(OH)3]=1.3��10��33 mol4��L��4�� �����ӣ��辭��ˮ������������ŷţ���ˮ�����������������̽��д�����
�����ӣ��辭��ˮ������������ŷţ���ˮ�����������������̽��д�����
 SO42����Cr3����H2O��δ��ƽ������ÿ����0.4mol Cr2O72��ת��__________mol e-��
SO42����Cr3����H2O��δ��ƽ������ÿ����0.4mol Cr2O72��ת��__________mol e-�� I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4�����Ϻ�ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�
I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4�����Ϻ�ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�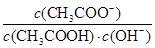 ��С
��С