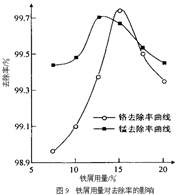
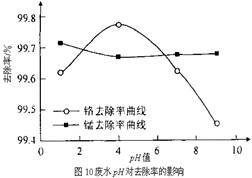
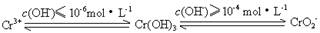��Ŀ����
��A��B��C��D��E���ֳ���������������±��е������γɵģ�
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ������B��CΪ��ɫ������ɫ�ܲ�������
���ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬֻ��A�зų���ɫ���壬ֻ��C��D�в�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɡ�
��1����������ʵ����գ�
��1��д��B��D�Ļ�ѧʽ��B ____��D ��
��2������lmol A����Һ�뺬l mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û����ﻯѧʽΪ__ _��
��3��C��������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��____ ___��
��4����A��Һ�м�����������ʯ��ˮ�������ӷ���ʽΪ_ ___��
��5����20mL 2mol/L C��Һ�м���30mL E��Һ����ַ�Ӧ��õ�0.78g��������E��Һ�����ʵ���Ũ�ȿ����� moI/L��
| ������ | K+ Na+ Cu2+ Al3+ |
| ������ | SO42�� HCO3�� NO3�� OH�� |
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ������B��CΪ��ɫ������ɫ�ܲ�������
���ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬֻ��A�зų���ɫ���壬ֻ��C��D�в�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɡ�
��1����������ʵ����գ�
��1��д��B��D�Ļ�ѧʽ��B ____��D ��
��2������lmol A����Һ�뺬l mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û����ﻯѧʽΪ__ _��
��3��C��������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��____ ___��
��4����A��Һ�м�����������ʯ��ˮ�������ӷ���ʽΪ_ ___��
��5����20mL 2mol/L C��Һ�м���30mL E��Һ����ַ�Ӧ��õ�0.78g��������E��Һ�����ʵ���Ũ�ȿ����� moI/L��
��12�֣�ÿ��2�֣���1��KNO3 CuSO4 ��2��Na2CO3
��3��Al3+��3H2O Al(OH)3(����) ��3H+
Al(OH)3(����) ��3H+
��4��2HCO3����Ca2+��2OH����CaCO3����2H2O��CO32�� ��5�� 1��5
��3��Al3+��3H2O
 Al(OH)3(����) ��3H+
Al(OH)3(����) ��3H+��4��2HCO3����Ca2+��2OH����CaCO3����2H2O��CO32�� ��5�� 1��5
��1��DΪ��ɫ��Һ��˵��D�к���ͭ���ӡ���E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⡣˵��E��ǿ�C�к��������ӣ�������ɫ��Ӧ������B��CΪ��ɫ��˵��B��C�к��м����ӣ�����ʵ��ܿ�֪��A�к���HCO3����C��D�к���SO42��,����A��̼�����ƣ�C���������أ�D������ͭ��E���������ƣ���B��D����Һ��ϣ�δ���������������ɣ�����B������ء�
��2��̼�����ƺ��������Ʒ�Ӧ�ķ���ʽ��HCO3- + OH- = CO32- +H2O�����Ըû�����Ļ�ѧʽ��̼���ơ�
��3��������������ˮ������������ӣ�ˮ�����������������壬������ˮ�е�������Ӷ���ˮ������ʽ��Al3����3H2O Al(OH)3��3H����
Al(OH)3��3H����
��4��ʯ��ˮ����ʱ��������ʱ̼��ơ�̼���ƺ�ˮ�����Է���ʽ��2HCO3����2OH����Ca2��=CaCO3����CO32����2H2O.
��5������������������������ܺ��������Ʒ�Ӧ�����Ը÷�Ӧ�ķ���ʽ��Al3����4OH��=AlO2����2H2O��
��6��20mL 2.0mol/LC��Һ�������ӵ����ʵ�����0.04mol����������������0.04mol��������������������3.12g����ʵ��������0.78g���������ʵ�����0.01mol��˵��������û����ȫת���������������������Ʋ��㣬����Ҫ0.03mol�������ƣ�������������Һ��Ũ����0.03mol��0.03L��1.0mol/L������������ƹ�������ƫ���������ɣ�����Ҫ����������0.04mol��3��0.03mol��0.15mol���������������Һ��Ũ����0.15mol��0.03L��5.0mol/L��
��2��̼�����ƺ��������Ʒ�Ӧ�ķ���ʽ��HCO3- + OH- = CO32- +H2O�����Ըû�����Ļ�ѧʽ��̼���ơ�
��3��������������ˮ������������ӣ�ˮ�����������������壬������ˮ�е�������Ӷ���ˮ������ʽ��Al3����3H2O
 Al(OH)3��3H����
Al(OH)3��3H������4��ʯ��ˮ����ʱ��������ʱ̼��ơ�̼���ƺ�ˮ�����Է���ʽ��2HCO3����2OH����Ca2��=CaCO3����CO32����2H2O.
��5������������������������ܺ��������Ʒ�Ӧ�����Ը÷�Ӧ�ķ���ʽ��Al3����4OH��=AlO2����2H2O��
��6��20mL 2.0mol/LC��Һ�������ӵ����ʵ�����0.04mol����������������0.04mol��������������������3.12g����ʵ��������0.78g���������ʵ�����0.01mol��˵��������û����ȫת���������������������Ʋ��㣬����Ҫ0.03mol�������ƣ�������������Һ��Ũ����0.03mol��0.03L��1.0mol/L������������ƹ�������ƫ���������ɣ�����Ҫ����������0.04mol��3��0.03mol��0.15mol���������������Һ��Ũ����0.15mol��0.03L��5.0mol/L��

��ϰ��ϵ�д�
 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ
 ��SO42-��CO32-,���ֻ�������������Ӿ�����ͬ���Ҿ�������ˮ���ֽ����Ƿֱ����0.1 mol��L-1����Һ����������ʵ�飺
��SO42-��CO32-,���ֻ�������������Ӿ�����ͬ���Ҿ�������ˮ���ֽ����Ƿֱ����0.1 mol��L-1����Һ����������ʵ�飺