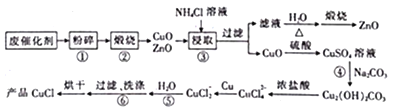
��Ŀ����
����Ŀ��25��ʱ����Ũ�Ⱦ�Ϊ0.1mol/L�������ͬ��HA��Һ��BOH ��Һ��ϣ�������Һ�������Ϊ100 mL,��Һ���������ҺpH �Ĺ�ϵ��ͼ��ʾ������˵����ȷ����

A. V1��ʾHA ��Һ�������V2��ʾBOH ��Һ�����
B. Ka( HA) ��Kb( BOH) ����������ȣ���Ϊ10-6
C. y��ʱ��c(B+)=c(A-)=c(OH-)=c(H+)
D. x��y��z ��ʾ����ʱˮ�ĵ���̶�:y>x=z
���𰸡�D
��������A. ����ͼ��HA��Һ��BOH��Һ��������ʱ����Һ��pH=7������V1��������Һ��pH������˵��V1��ʾBOH��Һ���������V2��ʾHA��Һ���������A����B. ����ͼ��HA��Һ��BOH��Һ��������ʱ����Һ��pH=7��˵����Һ�����ԣ�˵��Ka(HA)��Kb(BOH)��ȣ�0.1mol/L HA��ҺpH=3����Ka(HA)=![]() ��10-5����B����C. y��ʱ����Һ��pH=7��˵����Һ�����ԣ�����Ϊ�������ӵ�ˮ��̶���ͬ�����c(B+)=c(A-)��c(OH-)=c(H+)����C����D. x��z�������Ũ����ȣ���ΪKa(HA)��Kb(BOH)��ȣ���ˮ�ĵ�������Ƴ̶���ȣ�y���ε�Ũ�����ˮ�ĵ���Ĵٽ��̶��������ʱˮ�ĵ���̶�:y>x=z����D��ȷ����ѡD��
��10-5����B����C. y��ʱ����Һ��pH=7��˵����Һ�����ԣ�����Ϊ�������ӵ�ˮ��̶���ͬ�����c(B+)=c(A-)��c(OH-)=c(H+)����C����D. x��z�������Ũ����ȣ���ΪKa(HA)��Kb(BOH)��ȣ���ˮ�ĵ�������Ƴ̶���ȣ�y���ε�Ũ�����ˮ�ĵ���Ĵٽ��̶��������ʱˮ�ĵ���̶�:y>x=z����D��ȷ����ѡD��

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�����Ŀ���������Ʊ��������������Ҫԭ�ϣ����������������;�㷺���ش��������⣺
��1�����������Ĺ̵���ӦΪ��
N2(g)+O2(g)![]() 2NO(g)������������
2NO(g)������������
N2(g)+3H2(g)![]() 2NH3(g)������������
2NH3(g)������������
�����෴Ӧ�У�ij����A �����ϰ���÷�ѹp(A)����Ũ��c(A)����Ӧ��ƽ�ⳣ����Kp��ʾ����Ӧ����ƽ�ⳣ������ʽKp=_________________��
�ڷ�Ӧ�����ڲ�ͬ�¶��µ�ƽ�ⳣ��Kp���±���
�¶�/K | 298 | 473 | 673 |
Kp | 62(KPa)-2 | 6.2��10-5(KPa)-2 | 6.0��10-8(KPa)-2 |
��Ӧ(��) �Ħ�H________0�����������������=����
��2���ϳɰ���ҵ��ԭ��������������CO�Ժϳ����еĴ����к������ɡ�ͭϴ������ʵ�ֶ�ԭ�������ơ��йط�Ӧ�Ļ�ѧ����ʽ���£�
[Cu(NH3)2]Ac(aq)+CO(g) +NH3(g)![]() [Cu(NH3)3] ]Ac��CO(aq)
[Cu(NH3)3] ]Ac��CO(aq)
��H=-35 kJ��mol-1���û�ѧƽ���ƶ�ԭ�������ù�����������������Ϊ________________________��
��3�������ǹ�ҵ���������Ҫԭ�ϡ�T��ʱ��NH3��O2���ܷ������·�Ӧ��
��4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g) ��H= -907kJmol-1
4NO(g)+6H2O(g) ��H= -907kJmol-1
��4NH3(g)+3O2(g)![]() 2N2(g)+6H2O(g) ��H= -1269kJmol-1
2N2(g)+6H2O(g) ��H= -1269kJmol-1
���Т��Ǹ���Ӧ����Ҫ���ٸ���Ӧ�����NO�IJ��ʣ�������Ĵ�ʩ��___________________��
T��ʱN2��O2��Ӧ����NO���Ȼ�ѧ����ʽΪ________________________________��
��4��������ʵ���г����ü�ȩ���ⶨ��εĺ���������Ӧԭ�����£�
4NH4++6HCHO==(CH2)6N4H+��һԪ�ᣩ+3H++6H2O
ʵ�鲽�����£�
��ȡ�����Ʒ��Һa mL�������Թ����ļ�ȩ��Һ���ѳ�ȥ���е��ᣩ������1���ӣ�
�ڵ���1-2�η�̪��Һ����cmol��L-1������������Һ�ζ�����Һ�ʷۺ�ɫ�Ұ�����ڲ���ȥΪֹ����¼��������������Һ�������
���ظ����ϲ���2 �Σ�����ʵ��ƽ����������������ҺVmL��
����Ʒ�еĺ�����Ϊ____mg��L-1��������β��ʺ��ü�ȩ���ⶨ����������____________�����ţ���
a.NH4HCO3 b. (NH4)2SO4 c.NH4Cl d.CH3COONH4