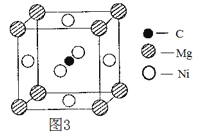
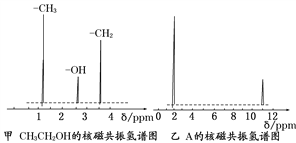
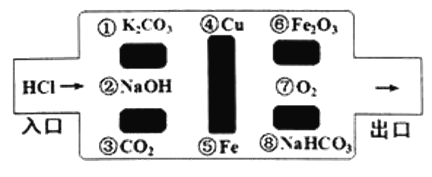
��Ŀ����
����Ŀ��CO(NH2)2(����)���Ʊ�������̼����������������������ȷ������Ź㷺��Ӧ�á��ش��������⣺
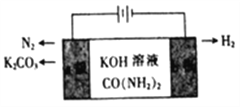
(1)������صļ�����Һ�Ʊ�������װ������ͼ��ʾ��a����________��ͨ��ʱ,������Χ��Һ��pH________(�����С��)�������ĵ缫��ӦʽΪ________��
(2)���غϳ�̼�����������ط�Ӧ��ƽ�ⳣ���Ķ���ֵ���¶ȵĹ�ϵ���£�
(��)2CH3OH(l)+CO(NH2)2(1)![]() CH3OCOOCH3(l)+2NH3(g)��H1
CH3OCOOCH3(l)+2NH3(g)��H1
(��)CH3OH(l)+CO(NH2)2(l)![]() CH3OCONH2(l)+NH3(g)��H2
CH3OCONH2(l)+NH3(g)��H2
(��)CH3OCONH2(l)+CH3OH(l)![]() CH3OCOOCH3(l)+NH3(g)��H3
CH3OCOOCH3(l)+NH3(g)��H3

�١�H1=_______(�á�H2�͡�H3��ʾ);ƽ�ⳣ���Ķ���lnK(��)=________[��lnK(��)��lnK(��)��ʾ]��
�ڶ��ڷ�Ӧ(��),�����¶ȣ���ѧƽ����________(���������桱)��Ӧ�����ƶ���
��Ϊ���CH3OCOOCH3�IJ���,�ɲ�ȡ�Ĵ�ʩ��________________________________(�о�2��)��
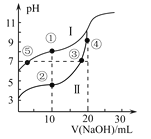
(3)����������NaClO2���û����Һ���������������������ú�NO��SO2�Ļ����������ʵ�顣���Һ��NaClO2������������NO��SO2���ѳ��ʹ�ϵ��ͼ��ʾ��

�ٺ�SO2������(��O2)ͨ��������Һ�õ�����Ϊ_________;NO2��������Һ��Ӧ������N2���������������д���÷�Ӧ�Ļ�ѧ����ʽ��_______________________________��
�����ô�������Һ����NO���ѳ��ʽ�ԼΪ15%,˵��NaClO2��������_____________��
��SO2���ѳ������DZ�NO�Ĵ�,��ԭ����____________________________(�о�2��)��
���𰸡� ���� ���� CO(NH2)2+8OH--6e-=CO32-+N2��+6H2O ��H2+��H3 lnK(��)+lnK(��) �� �����¶�,��Сѹǿ (NH4)2SO4 6NO2+4CO(NH2)2=7N2+4CO2+8H2O ��NO����Ϊ������ˮ��NO2�ȸ������� SO2���ܽ�ȱ�NO�Ĵ�,NO��CO(NH2)2��Ӧ�Ļ�ܱ�SO2�Ĵ�
��������(1)��������������������������a����������ͨ��ʱ,����ˮ��������������ӵõ��Ӳ�������������������Ũ��������Χ��Һ��pH��������). CO(NH2)2�ڼ��������µõ��Ӳ���CO32-��N2���缫��ӦʽΪCO(NH2)2+8OH--6e-=CO32-+N2��+6H2O��(2) ����֪��(��)CH3OH(l)+CO(NH2)2(l)![]() CH3OCONH2(l)+NH3(g)��H2
CH3OCONH2(l)+NH3(g)��H2
(��)CH3OCONH2(l)+CH3OH(l)![]() CH3OCOOCH3(l)+NH3(g)��H3
CH3OCOOCH3(l)+NH3(g)��H3
���ݸ�˹���ɣ���(��)+ (��)�÷�Ӧ(��)2CH3OH(l)+CO(NH2)2(1)![]() CH3OCOOCH3(l)+2NH3(g)��H1=��H2+��H3����K=(��)= K(��) K(��)����lnK(��)= lnK(��)+lnK(��)���ڸ���ͼ�����߱仯��֪�������¶�lnK(��)������K(��)����ѧƽ��������Ӧ�����ƶ�����Ϊ���CH3OCOOCH3�IJ���,��ʹ��Ӧ(��)CH3OCONH2(l)+CH3OH(l)
CH3OCOOCH3(l)+2NH3(g)��H1=��H2+��H3����K=(��)= K(��) K(��)����lnK(��)= lnK(��)+lnK(��)���ڸ���ͼ�����߱仯��֪�������¶�lnK(��)������K(��)����ѧƽ��������Ӧ�����ƶ�����Ϊ���CH3OCOOCH3�IJ���,��ʹ��Ӧ(��)CH3OCONH2(l)+CH3OH(l)![]() CH3OCOOCH3(l)+NH3(g)������Ӧ�����ƶ����÷�ӦΪ���������������ȷ�Ӧ���ɲ�ȡ�Ĵ�ʩ�������¶�,��Сѹǿ���ٺ�SO2������(��O2)ͨ��������Һ�õ�����Ϊ������(NH4)2SO4;NO2��������Һ��Ӧ������N2���������������������̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��6NO2+4CO(NH2)2=7N2+4CO2+8H2O�������ô�������Һ����NO���ѳ��ʽ�ԼΪ15%,˵��NaClO2�������ǽ�NO����Ϊ������ˮ��NO2�ȸ��������SO2���ѳ������DZ�NO�Ĵ�,��ԭ����SO2���ܽ�ȱ�NO�Ĵ�,NO��CO(NH2)2��Ӧ�Ļ�ܱ�SO2�Ĵ�
CH3OCOOCH3(l)+NH3(g)������Ӧ�����ƶ����÷�ӦΪ���������������ȷ�Ӧ���ɲ�ȡ�Ĵ�ʩ�������¶�,��Сѹǿ���ٺ�SO2������(��O2)ͨ��������Һ�õ�����Ϊ������(NH4)2SO4;NO2��������Һ��Ӧ������N2���������������������̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��6NO2+4CO(NH2)2=7N2+4CO2+8H2O�������ô�������Һ����NO���ѳ��ʽ�ԼΪ15%,˵��NaClO2�������ǽ�NO����Ϊ������ˮ��NO2�ȸ��������SO2���ѳ������DZ�NO�Ĵ�,��ԭ����SO2���ܽ�ȱ�NO�Ĵ�,NO��CO(NH2)2��Ӧ�Ļ�ܱ�SO2�Ĵ�