��Ŀ����
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã���ش�
��1����һ������ĺ����ܱ������У��������»�ѧ��Ӧ��N2��g��+3H2��g�� 2NH3��g��
2NH3��g��
�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
����������⣺
�ٱȽ�K1��K2�Ĵ�С��K1
���жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������
A��2v��H2��������=3v��NH3�����棩 B��v��N2��������=3v��H2�����棩
C��������ѹǿ���ֲ��� D�����������ܶȱ��ֲ���
��2�������£�N2H6Cl2����һ����Ҫ�Ļ���ԭ�ϣ��������ӻ����������ˮ����Һ�����ԣ�ˮ��ԭ����NH4Cl���ƣ�
��д�������µ�һ��ˮ�ⷴӦ�����ӷ���ʽ
��������ˮ��Һ������Ũ�ȵ�����˳����ȷ����
A��c��Cl-����c��N2H62+����c��H+����c��OH-��
B��c��Cl-����c��[N2H5?H2O+]����c��H+����c��OH-��
C��c��N2H62+��+c��[N2H5?H2O+]��+c��H+��=c��Cl-��+c��OH-��
D��c��N2H62+����c��Cl-����c��H+����c��OH-��
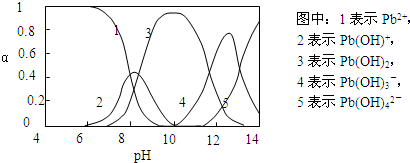
��ˮ�����ؽ���Ǧ����Ⱦ���ⱸ�ܹ�ע�������ϵ�֪Pb4+���к�ǿ�������ԣ�ˮ��Ǧ�Ĵ�����̬��Ҫ��Pb2+��Pb��OH��+��Pb��OH��2����ˮ���ܽ��С����Pb��OH��3-��Pb��OH��42-������̬�����ʵ����ķ���������ҺpH�仯�Ĺ�ϵ����ͼ��ʾ��
��1��Pb��NO3��2��Һ�У�c��Pb2+��/c��NO3-��
��2����Pb��NO3��2��Һ�μ����ᣬ��Һ��c��Pb2+��/c��NO3-��û�б������С���г������ɣ������ɵij�������Ϊ
��3����Pb��NO3��2��Һ�еμ�NaOH��Һ����ҺҲ����ǣ���pHԼΪ
��1����һ������ĺ����ܱ������У��������»�ѧ��Ӧ��N2��g��+3H2��g��
 2NH3��g��
2NH3��g���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
| t/K | 298 | 398 | 498 | �� |
| K/��mol?L-1��2 | 4.1��106 | K1 | K2 | �� |
�ٱȽ�K1��K2�Ĵ�С��K1
��
��
K2�����������=�������������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������
AC
AC
������ţ���A��2v��H2��������=3v��NH3�����棩 B��v��N2��������=3v��H2�����棩
C��������ѹǿ���ֲ��� D�����������ܶȱ��ֲ���
��2�������£�N2H6Cl2����һ����Ҫ�Ļ���ԭ�ϣ��������ӻ����������ˮ����Һ�����ԣ�ˮ��ԭ����NH4Cl���ƣ�
��д�������µ�һ��ˮ�ⷴӦ�����ӷ���ʽ
N2H62++H2O [N2H5?H2O]++H+
[N2H5?H2O]++H+
 [N2H5?H2O]++H+
[N2H5?H2O]++H+N2H62++H2O [N2H5?H2O]++H+
[N2H5?H2O]++H+
�� [N2H5?H2O]++H+
[N2H5?H2O]++H+��������ˮ��Һ������Ũ�ȵ�����˳����ȷ����
A
A
������ţ���A��c��Cl-����c��N2H62+����c��H+����c��OH-��
B��c��Cl-����c��[N2H5?H2O+]����c��H+����c��OH-��
C��c��N2H62+��+c��[N2H5?H2O+]��+c��H+��=c��Cl-��+c��OH-��
D��c��N2H62+����c��Cl-����c��H+����c��OH-��
��ˮ�����ؽ���Ǧ����Ⱦ���ⱸ�ܹ�ע�������ϵ�֪Pb4+���к�ǿ�������ԣ�ˮ��Ǧ�Ĵ�����̬��Ҫ��Pb2+��Pb��OH��+��Pb��OH��2����ˮ���ܽ��С����Pb��OH��3-��Pb��OH��42-������̬�����ʵ����ķ���������ҺpH�仯�Ĺ�ϵ����ͼ��ʾ��

��1��Pb��NO3��2��Һ�У�c��Pb2+��/c��NO3-��
��
��
1/2�����������=����������������2����Pb��NO3��2��Һ�μ����ᣬ��Һ��c��Pb2+��/c��NO3-��û�б������С���г������ɣ������ɵij�������Ϊ
PbCl2
PbCl2
����3����Pb��NO3��2��Һ�еμ�NaOH��Һ����ҺҲ����ǣ���pHԼΪ
10
10
ʱ���ɳ�����࣬�����μ�NaOH��Һ�������ϵ������壮pH=13ʱ�������ϵ�з�������Ҫ��Ӧ�����ӷ���ʽΪ��Pb��OH��3-+OH-=Pb��OH��42-
Pb��OH��3-+OH-=Pb��OH��42-
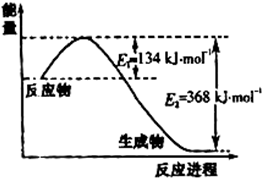
����������1���ٸ��ݺϳɰ���ӦΪ���ȷ�Ӧ���¶ȶԻ�ѧƽ���Ӱ��������K�ı仯��
�ڸ��ݻ�ѧƽ����������ȡ��롰�������ж��Ƿ�ﵽ��ѧƽ�⣻
��2���ٸ������ӻ����������ˮ����Һ�����ԣ�ˮ��ԭ����NH4Cl���ƣ���N2H62+ˮ����H2O�������ɵ�OH-��
���������µĻ�ѧʽΪN2H6Cl2��ˮ��ʹ��Һ����������������Ũ�ȵĴ�С��ϵ��
��1������ˮ��Ǧ�Ĵ�����̬��Ҫ��Pb2+��Pb��OH��+��Pb��OH��2����ˮ���ܽ��С����Pb��OH��3-��Pb��OH��42-��������
��2�����ݼ������ᣬpH��С�����ͼ��Pb2+���࣬c��Pb2+��/c��NO3-��û�б������С���г������ɣ���ֻ����Pb2+���������ӽ�����ɳ�����
��3����ͼ���Կ�����pH=10ʱ�������3��֪������࣬ΪPb��OH��2�������������������ƣ�������4��5��֪��pH=13ʱ����Pb��OH��3-ת��ΪPb��OH��42-�����ӷ�Ӧ��
�ڸ��ݻ�ѧƽ����������ȡ��롰�������ж��Ƿ�ﵽ��ѧƽ�⣻
��2���ٸ������ӻ����������ˮ����Һ�����ԣ�ˮ��ԭ����NH4Cl���ƣ���N2H62+ˮ����H2O�������ɵ�OH-��
���������µĻ�ѧʽΪN2H6Cl2��ˮ��ʹ��Һ����������������Ũ�ȵĴ�С��ϵ��
��1������ˮ��Ǧ�Ĵ�����̬��Ҫ��Pb2+��Pb��OH��+��Pb��OH��2����ˮ���ܽ��С����Pb��OH��3-��Pb��OH��42-��������
��2�����ݼ������ᣬpH��С�����ͼ��Pb2+���࣬c��Pb2+��/c��NO3-��û�б������С���г������ɣ���ֻ����Pb2+���������ӽ�����ɳ�����
��3����ͼ���Կ�����pH=10ʱ�������3��֪������࣬ΪPb��OH��2�������������������ƣ�������4��5��֪��pH=13ʱ����Pb��OH��3-ת��ΪPb��OH��42-�����ӷ�Ӧ��
����⣺��1������÷�ӦΪ���ȷ�Ӧ�����¶����ߣ���ѧƽ�����淴Ӧ�����ƶ����������¶�K��С����K1��K2���ʴ�Ϊ������
���ɻ�ѧƽ���������֪��Aת��Ϊ��2v��H2��������=3v��NH3��������=3v��NH3�����棩�������淴Ӧ������ȣ��ﵽƽ�⣬��Bת��Ϊͬһ���ʲ����������淴Ӧ������ȣ�
�÷�Ӧ�Ƿ�Ӧǰ�����ʵ������ȵķ�Ӧ����ѹǿ�����˵����ѧ��Ӧ�ﵽƽ�⣬��D������ʼ�ղ��䣬�ܱ�������������䣬����������ܶ�ʼ�ղ��䣬������Ϊ�ж�ƽ������ݣ��ʴ�Ϊ��AC��
��2������ˮ����NH4Cl���ƣ���N2H62+ˮ����H2O�������ɵ�OH-����ˮ�����ӷ�ӦΪN2H62++H2O [N2H5?H2O]++H+���ʴ�Ϊ��N2H62++H2O
[N2H5?H2O]++H+���ʴ�Ϊ��N2H62++H2O [N2H5?H2O]++H+��
[N2H5?H2O]++H+��
���������µĻ�ѧʽΪN2H6Cl2����c��Cl-����c��N2H62+������ˮ�������ԣ���c��H+����c��OH-������ˮ��ij̶Ⱥ�������c��N2H62+����c��H+����������Ũ�ȵĴ�С��ϵΪ
c��Cl-����c��N2H62+����c��H+����c��OH-������A��ȷ����ȻD������ˮ�ⷽ��ʽ��֪c��[N2H5?H2O+]��=c��H+������B������ȷ�ĵ���غ�ʽΪ
2c��N2H62+��+c��[N2H5?H2O+]��+c��H+��=c��Cl-��+c��OH-������C���ʴ�Ϊ��A��
��1����Pb����Һ���ж��ִ�����̬����Pb��NO3��2��Һ�У�c��Pb2+��/c��NO3-����1/2���ʴ�Ϊ������
��2���ɼ������ᣬpH��С�����ͼ��֪Pb2+���࣬��c��Pb2+��/c��NO3-��û�б������С���г������ɣ���ֻ����Pb2+��Cl-���ӽ�����ɳ���PbCl2���ʴ�Ϊ��PbCl2��
��3����ͼ�е�����3��֪��pH=10ʱ���ɳ�����࣬�������������ƣ�������4��5��֪��pH=13ʱ����Pb��OH��3-ת��ΪPb��OH��42-�����ӷ�Ӧ�����ӷ�ӦΪPb��OH��3-+OH-=Pb��OH��42-��
�ʴ�Ϊ��10��Pb��OH��3-+OH-=Pb��OH��42-��
���ɻ�ѧƽ���������֪��Aת��Ϊ��2v��H2��������=3v��NH3��������=3v��NH3�����棩�������淴Ӧ������ȣ��ﵽƽ�⣬��Bת��Ϊͬһ���ʲ����������淴Ӧ������ȣ�
�÷�Ӧ�Ƿ�Ӧǰ�����ʵ������ȵķ�Ӧ����ѹǿ�����˵����ѧ��Ӧ�ﵽƽ�⣬��D������ʼ�ղ��䣬�ܱ�������������䣬����������ܶ�ʼ�ղ��䣬������Ϊ�ж�ƽ������ݣ��ʴ�Ϊ��AC��
��2������ˮ����NH4Cl���ƣ���N2H62+ˮ����H2O�������ɵ�OH-����ˮ�����ӷ�ӦΪN2H62++H2O
 [N2H5?H2O]++H+���ʴ�Ϊ��N2H62++H2O
[N2H5?H2O]++H+���ʴ�Ϊ��N2H62++H2O [N2H5?H2O]++H+��
[N2H5?H2O]++H+�� ���������µĻ�ѧʽΪN2H6Cl2����c��Cl-����c��N2H62+������ˮ�������ԣ���c��H+����c��OH-������ˮ��ij̶Ⱥ�������c��N2H62+����c��H+����������Ũ�ȵĴ�С��ϵΪ
c��Cl-����c��N2H62+����c��H+����c��OH-������A��ȷ����ȻD������ˮ�ⷽ��ʽ��֪c��[N2H5?H2O+]��=c��H+������B������ȷ�ĵ���غ�ʽΪ
2c��N2H62+��+c��[N2H5?H2O+]��+c��H+��=c��Cl-��+c��OH-������C���ʴ�Ϊ��A��
��1����Pb����Һ���ж��ִ�����̬����Pb��NO3��2��Һ�У�c��Pb2+��/c��NO3-����1/2���ʴ�Ϊ������
��2���ɼ������ᣬpH��С�����ͼ��֪Pb2+���࣬��c��Pb2+��/c��NO3-��û�б������С���г������ɣ���ֻ����Pb2+��Cl-���ӽ�����ɳ���PbCl2���ʴ�Ϊ��PbCl2��
��3����ͼ�е�����3��֪��pH=10ʱ���ɳ�����࣬�������������ƣ�������4��5��֪��pH=13ʱ����Pb��OH��3-ת��ΪPb��OH��42-�����ӷ�Ӧ�����ӷ�ӦΪPb��OH��3-+OH-=Pb��OH��42-��
�ʴ�Ϊ��10��Pb��OH��3-+OH-=Pb��OH��42-��
�����������ѶȽϴ��黯ѧƽ���Ӱ�����ء�ƽ����ж���ˮ��ƽ�⡢���ӵ�Ũ�ȴ�С�ıȽϡ����ӷ�Ӧ�ȣ���ȷͼ������ü�����ͼ�������������ǽ������ѵ㣮

��ϰ��ϵ�д�
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
�����Ŀ
 ���ǵ����Ϻ����ḻ��һ��Ԫ�أ��䵥�ʼ��������ڹ�ũҵ������������������Ҫ���ã�
���ǵ����Ϻ����ḻ��һ��Ԫ�أ��䵥�ʼ��������ڹ�ũҵ������������������Ҫ���ã�
 ���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã���ش�
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã���ش�