��Ŀ����
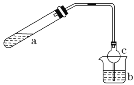
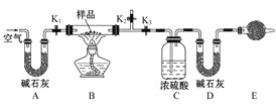
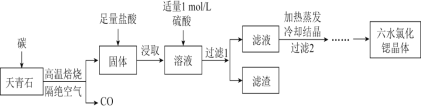
����Ŀ����(Sr)Ϊ��������IIA��Ԫ�أ��仯������ˮ�Ȼ���(SrCl26H2O)��ʵ������Ҫ�ķ����Լ�����ҵ�ϳ�������ʯ(��Ҫ�ɷ�ΪSrSO4)Ϊԭ���Ʊ�������������ͼ��
��֪���پ������ȡ�����Һ�г�����Sr2+��Cl-�⣬����������Ba2+���ʡ�
��BaSO4���ܶȻ�����Ϊ2.2��10-10��SrSO4���ܶȻ�����Ϊ3.3��10-7��
��SrCl26H2O��Ħ������Ϊ267g/mol��
(1)��ҵ������ʯ����ǰӦ����ĥ���飬��Ŀ����__��
(2)��ҵ������ʯ�����������±���ʱ����0.5molSrSO4��ֻ��SԪ�ر���ԭ����ת����4mol���ӡ���÷�Ӧ�Ļ�ѧ����ʽΪ__��
(3)��ȡ����������Ŀ����___�������ӷ���ʽ��ʾ___��
(4)��Ʒ���ȼ�⣺��ȡ2.000g��Ʒ�ܽ�������ˮ�У������м��뺬AgNO30.01mol��AgNO3��Һ����Һ�г�Cl-�⣬����������Ag+��Ӧ�����ӣ���Cl-��ȫ��������1��2�κ�Fe3+����Һ��ָʾ������0.2000mol/L��NH4SCN����Һ�ζ�ʣ���AgNO3��ʹʣ���Ag+��AgSCN��ɫ��������ʽ��������֪��SCN-����Ag+��Ӧ��
�ٵζ���Ӧ�ﵽ�յ��������__��
�����ζ�������ȥ����Ũ�ȵ�NH4SCN��Һ20.00mL�����Ʒ��SrCl26H2O�������ٷֺ���Ϊ___(���г�����ʽ���������)��
(5)��SrCl26H2O������ȡ��ˮ�Ȼ��ȵ���Ҫ�������˾ƾ��ơ������ǡ����ż��⣬����___��
���𰸡����ӷ�Ӧ��ĽӴ���������ѧ��Ӧ���� SrSO4+4C![]() SrS+4CO�� ��ȥ����Ba2+ SO
SrS+4CO�� ��ȥ����Ba2+ SO![]() +Ba2+=BaSO4�� ���������1��NH4SCN��Һʱ����Һ����ɫ��Ϊ��ɫ����30s�ڲ���ɫ
+Ba2+=BaSO4�� ���������1��NH4SCN��Һʱ����Һ����ɫ��Ϊ��ɫ����30s�ڲ���ɫ ![]() ��100% ����
��100% ����
��������
������ʯ(��Ҫ�ɷ�ΪSrSO4)Ϊԭ���Ʊ���ˮ�Ȼ���(SrCl26H2O)�������̿�֪������ʯ��̼�����������±�������CO��SrS��SrS�������ܽ��������Һ�г�����Sr2+��Cl-�⣬����������Ba2+���ʣ�Ȼ��������������ᱵ���������Թ��˺�����Ϊ���ᱵ����Һ����Ҫ��SrCl2����������������ȴ�ᾧ�����˵õ�SrCl26H2O���ݴ˷������
(1)��ĥ�����Ŀ�������ӷ�Ӧ��ĽӴ���������ѧ��Ӧ���ʣ�
(2)��0.5molSrSO4��ֻ��S����ԭ��ת����4mol���ӣ���1mol SrSO4��Ӧ����ת��8mol�����������ȵĻ�ԭ����ΪSrS����÷�Ӧ�Ļ�ѧ����ʽΪSrSO4+4C![]() SrS+4CO����
SrS+4CO����
(3)���ᱵ���ܶȻ������������ȵ��ܶȻ�����С�ö࣬����HCl�ܽ�SrS�����Һ�м��������Ŀ���dz�ȥ��Һ��Ba2+���ʣ���ӦΪ��SO42-+Ba2+=BaSO4����
(4)����֪��SCN-����Ag+��Ӧ��NH4SCN��ʣ���Ag+����γ�AgSCN��ɫ������������Һ�е�Ag+ȫ���������ټ���NH4SCN�������SCN-�ͻ���Fe3+���������ʹ��Һ��Ϊ��ɫ����˵��������1��NH4SCN��Һ�ﵽ�յ�ʱ����Һ����ɫ��Ϊ��ɫ����30 s����ɫ��
��n(NH4SCN)=0.2000mol/L��0.02L=0.2��0.02mol��Ag+��AgSCN��ɫ��������ʽ������������Һ�й�����Ag+�����ʵ���Ϊ��n(Ag+)=0.2��0.02mol������Cl-��Ӧ��Ag+�����ʵ���n(Ag+)=0.01mol-0.2��0.02mol =(0.01-0.2��0.02)mol��2.000g��Ʒ��SrCl26H2O�����ʵ���n(SrCl26H2O)=![]() ��n(Ag+)=
��n(Ag+)=![]() mol��2.000g��Ʒ��SrCl26H2O������m(SrCl26H2O)=
mol��2.000g��Ʒ��SrCl26H2O������m(SrCl26H2O)=![]() mol��267 g/mol=
mol��267 g/mol=![]() g�����Բ�Ʒ����Ϊ��
g�����Բ�Ʒ����Ϊ�� ��100%=
��100%=![]() ��100%��
��100%��
(5)��SrCl26H2O������ȡ��ˮ�Ȼ��ȵ������оƾ��ơ������ǡ����żܡ�������

 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�