��Ŀ����
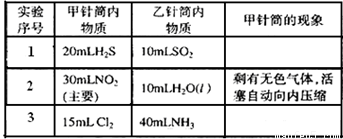
����С������9�֣���ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ�����Իش��������⣺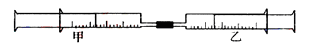

��1��ʵ��1����Ͳ�ڵ������ǣ���___________���ɣ���Ͳ����________�ƶ��������⡢���ڡ���������Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��___________��Һ�С�
��2��ʵ��2�У����е�3mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������_______��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽΪ____________________��
��3��ʵ��3�У���֪��3Cl2��2NH3===N2��6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ��Ϊ____________�������Ͳ��ʣ����������ԼΪ________mL��
��1����ɫ���壻���ڣ�NaOH����ÿ��1�֣�
��2��NO ��1�֣��� 3NO2+H2O=2HNO3��NO��2�֣���
��3����ɫ��1�֣��� 5��2�֣�
����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ