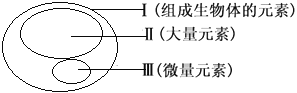
��Ŀ����
����Ŀ��һ�������̿�(��Ҫ�ɷ�MnO2)�ͻ�����(��Ҫ�ɷ�FeS2)��ȡMnSO4��H2O�����յ�����Ĺ����������£�

�ش��������⣺
(1)��������ȡʱ������S��MnSO4��Fe2(SO4)3�Ļ�ѧ����ʽΪ______________________��
(2)����������������Һ���ܺ���Fe2+����ȥFe2+���õ�������______________________���������轫��Һ��������Ȼ���ڲ��Ͻ����¼�������pH4~5���ټ������һ��ʱ�䣬�������������Ŀ����___________����������������Ϊ___________(�ѧʽ)��
(3)����������90~100���½��У��÷�Ӧ�Ļ�ѧ����ʽΪ______________________��
(4)�ⶨ��ƷMnSO4��H2O�ķ���֮һ�ǣ�ȷ��ȡag��Ʒ����ƿ�У���������ZnO��H2O��У�Ȼ���� c mol��L��1 KMnO4����Һ�ζ���dz��ɫ�Ұ���Ӳ��ʣ����ı���ҺVmL���ζ���Ӧ�����ӷ���ʽΪ_________________________________����Ʒ��Mn2+����������Ϊw(Mn2+)=_________________________________��
���𰸡�3MnO2 +2FeS2+6H2SO4=3MnSO4+Fe2(SO4)3+4S��+6H2O �������̿�ۻ�H2O2��Һ �ƻ�Fe(OH)3���岢ʹ�������������ڹ��˷��� Fe(OH)3 (NH4)2Sx+1![]() 2NH3+H2S + xS�� 2MnO4��+3Mn2++2H2O
2NH3+H2S + xS�� 2MnO4��+3Mn2++2H2O![]() 5MnO2��+4H+
5MnO2��+4H+ ![]()
��������
��1���������̣���������ΪMnO2��FeS2��H2SO4��������ΪS��MnSO4��Fe2(SO4)3������ѧ����ʽΪ3MnO2��2FeS2��6H2SO4=3MnSO4��Fe2(SO4)3��3S����6H2O��
��2����ȥFe2�����������������ʣ�Fe2���Ի�ԭ��Ϊ������Ϊ��Ҫ�Ʊ�MnSO4��H2O��MnO2����������������˿��Լ���MnO2�����̿�ۣ�H2O2Ϊ��ɫ�����������Ҳ���Լ���H2O2��Һ���������̣�������dz�ȥ��Ԫ�أ�������轫��Һ��������Ȼ���ڲ��Ͻ����¼�������pH4~5���ټ������һ��ʱ�䣬�������������Ŀ�����ƻ�Fe(OH)3���岢ʹ�������������ڹ��˷��룻�������������ΪFe(OH)3��
��3���������̣�����߷����ֽⷴӦ�����ɲ�ƷS���ֽ��ˮ��������(NH4)2S���Ƴ�����߷ֽⷴӦ����������NH3��H2S�����������90��100��ʱ��������ѧ����ʽΪ(NH4)2Sx��1![]() 2NH3����H2S����xS����
2NH3����H2S����xS����
��4��������ؾ���ǿ�����ԣ��μ��յ���Һ��ɫ��Ϊdz��ɫ��˵��������زμ�������ԭ��Ӧ��������Ϣ���Ƴ�MnSO4Ϊ��ԭ�ԣ����ζ���Ӧ�����ӷ���ʽΪ2MnO4����3Mn2����2H2O![]() 5MnO2����4H�����������ӷ���ʽ��m(Mn2��)=
5MnO2����4H�����������ӷ���ʽ��m(Mn2��)=![]() ��w(Mn2��)=
��w(Mn2��)=![]() =8.25cV��10��2/a��100%��
=8.25cV��10��2/a��100%��

 ��У����ϵ�д�
��У����ϵ�д�