题目内容
【题目】工业上用含锰废料(主要成分MnO2,含有少量Fe2O3、Al2O3、CuO、CaO等)与烟气脱硫进行联合处理并制备MnSO4的流程如下:
已知:25℃时,部分氢氧化物的溶度积常数(Ksp)如下表所示。
氢氧化物 | Al(OH)3 | Fe(OH)3 | Cu(OH)2 | Mn(OH)2 |
Ksp | 1.3×10-33 | 4.0×10-38 | 2.2×10-20 | 1.9×10-14 |
请回答:
(1)沉淀1的化学式为__________________。
(2)室温下,调节pH为5。试通过计算说明此时Al3+、Fe3+已沉淀完全,理由是_________。 “净化”时,加入(NH4)2S的作用为___________________。
(3)“酸化、还原”中,发生的所有氧化还原反应的离子方程式为__________________。
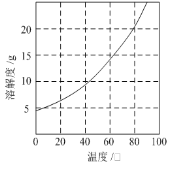
(4)已知:滤液3中除MnSO4外,还含有少量(NH4)2SO4。(NH4)2SO4、MnSO4的溶解度曲线如下图所示。据此判断,操作“I”应为蒸发浓缩、____________、洗涤、干燥。

(5)工业上可用电解酸性MnSO4溶液的方法制备MnO2,其阳极反应式为________________。
(6)25.35 g MnSO4·H2O样品受热分解过程的热重曲线(样品质量随温度变化的曲线)如下图所示。1150℃时,反应的化学方程式为___________________。
 。
。
【答案】CaSO4pH=5时,[Al3+]=1.3×10-33/(10-9)3=1.3×10-6 <×10-5,说明Al3+已经沉淀完全。Fe(OH)3的Ksp更小,因此Fe3+也已沉淀完全除去Cu2+MnO2+SO2=Mn2++SO42- 、 Fe2O3+SO2+2H+=2Fe2++SO42-+H2O趁热过滤Mn2++2H2O-2e-=MnO2+4H+3MnO2 ![]() Mn3O4+O2↑
Mn3O4+O2↑
【解析】
含锰废料(主要成分MnO2,含有少量Fe2O3、Al2O3、CuO、CaO等)与含有二氧化硫烟气、稀硫酸溶解,发生SO2+MnO2═Mn2++SO42-,Fe2O3+SO2+2H+=2Fe2++SO42-+H2O,Al2O3+6H+=2Al3++3H2O,CuO+2H+=Cu2++H2O,CaO+2H++SO42-=CaSO4↓+H2O,沉淀1为CaSO4,滤液1 金属阳离子为Mn2+、Fe2+、Al3+、Cu2+,加入双氧水氧化Fe2+为Fe3+,调节pH为5沉淀Fe3+、Al3+,沉淀2为Al(OH)3和Fe(OH)3,滤液2金属阳离子为Mn2+、Cu2+,加入(NH4)2S沉淀Cu2+,滤液3中除MnSO4外,还含有少量(NH4)2SO4,将滤液3蒸发浓缩、趁热过滤、洗涤、干燥制备MnSO4,据此分析解答。
(1)含锰废料中氧化钙与稀硫酸反应:CaO+2H++SO42-=CaSO4↓+H2O,沉淀1为CaSO4;
(2)pH=5时,c(Al3+)=1.3×10-33/(10-9)3=1.3×10-6 <×10-5,说明Al3+已经沉淀完全。Fe(OH)3的Ksp更小,因此Fe3+也已沉淀完全;(NH4)2S的作用沉淀Cu2+使Cu2+转化为CuS沉淀;
(3)“酸化、还原”中,发生的所有氧化还原反应的离子方程式为:SO2+MnO2═Mn2++SO42-,Fe2O3+SO2+2H+=2Fe2++SO42-+H2O;
(4)由图可知(NH4)2SO4的溶解度随温度的升高而增大,MnSO4的溶解度随温度的升高减小,故将滤液3蒸发浓缩、趁热过滤、洗涤、干燥制备MnSO4;
(5)用电解酸性MnSO4溶液的方法制备MnO2,阳极发生氧化反应,反应式为:Mn2++2H2O-2e-=MnO2+4H+;
(6)25.35g MnSO4H2O样品n(锰)=n(MnSO4H2O)=0.15mol,其中n(H2O)=0.15mol,m(H2O)=2.7g,300℃时,所得固体质量为22.65g,减少的质量为2.7g,则说明该段失去结晶水,此时固体为:MnSO4;300℃时,固体为:MnSO4,受热分解生成锰的氧化物和硫的氧化物0.15mol,850℃时,固体质量由22.65g减少到为13.05g,减少的质量为9.6g,则硫氧化物的相对质量为64,故为二氧化硫,则此时的固体为MnO2,1150℃时固体为为二氧化锰分解所得,锰元素质量守恒,则m(锰)=n(锰)×55=8.25g,则氧化物中m(O)=11.45g-8.25g=3.2g,n(O)=0.2mol,故n(Mn):n(O)=0.15:0.2=3:4,则该氧化物为:Mn3O4,故反应为:3MnO2 ![]() Mn3O4+O2↑。
Mn3O4+O2↑。

 尖子生新课堂课时作业系列答案
尖子生新课堂课时作业系列答案 英才计划同步课时高效训练系列答案
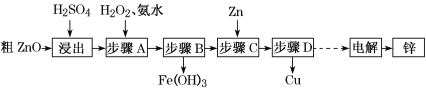
英才计划同步课时高效训练系列答案【题目】工业上利用粗ZnO(含FeO、CuO)制取金属锌的流程如下(部分步骤省略):
已知:几种离子形成氢氧化物沉淀时的pH如下表:
待沉淀的离子 | Fe2+ | Fe3+ | Zn2+ | Cu2+ |
开始沉淀时pH | 6.3 | 1.5 | 6.2 | 5.2 |
沉淀完全时pH | 9.7 | 3.2 | 8.0 | 6.4 |
请回答下列问题:
(1)酸浸粗氧化锌的过程中,为了加快酸浸速率,可采取的措施有______________ (写出一点即可)。
(2)步骤A的目的是将Fe2+氧化为Fe3+,并全部形成Fe(OH)3沉淀,为了暂不形成Cu(OH)2、Zn(OH)2,该步骤需控制溶液pH的范围是______________,该步骤中加入H2O2发生反应的离子方程式为_______________________________________。
(3)步骤D的操作名称为________________________________________________。
(4)由粗ZnO制取单质锌的另一种方法是将粗ZnO(含FeO、CuO)溶于NaOH溶液,ZnO全
部转化为Na2[Zn(OH)4]溶液,该反应的化学方程式为_________________________;然后将FeO、CuO过滤除去;再用惰性电极电解该滤液,阳极上逸出无色无味气体,阴极上析出锌,则阴极电极反应式为_____________________________________________。