��Ŀ����
ijѧ����NaHCO3��KHCO3��ɵĻ�������ʵ�飬�����������(ÿ�μ������������ʵ���Ũ�����)�����з�����ȷ����(����)
| ����/mL | 50 | 50 | 50 |
| m(�����)/g | 9.2 | 15.7 | 27.6 |
| V(CO2)(��״��)/L | 2.24 | 3.36 | 3.36 |
A����������ʵ���Ũ��Ϊ3.0 mol��L��1
B���������NaHC O3����������Ϊ54.3%
O3����������Ϊ54.3%
C��9.2 g�������KHCO3���� �ʵ���Ϊ0.05 mol
�ʵ���Ϊ0.05 mol
D��15.7 g�����ǡ����������ȫ��Ӧ
������ѡAC����m��27.6 gʱ�����������㣬��������������������������H����HCO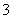 ===CO2����H2O֪��n(HCl)��3.36 L��22.4 L��mol��1��0.15 mol��c(HCl)��0.15 mol��0.05 L�� 3.0 mol��L��1��A����ȷ����m��9.2 gʱ�������������9.2 g������У�NaHCO3��KHCO3�����ʵ����ֱ�Ϊn(NaHCO3)��n(KHCO3)�����У�n(NaHCO3)��n(KHCO3)��0.1 mol,84 g��mol��1 ��n(NaHCO3)��100 g��mol��1��n(KHCO3)��9.2 g��������ʽ��ã�n(NaHCO3)��n(KHCO3)��0.05 mol��C����ȷ��NaHCO3����������С��KHCO3������������B���ȷ������������ʱ������3.36 L�����������������Ϊ9.2 g��1.5��13.8 g��D���ȷ��
===CO2����H2O֪��n(HCl)��3.36 L��22.4 L��mol��1��0.15 mol��c(HCl)��0.15 mol��0.05 L�� 3.0 mol��L��1��A����ȷ����m��9.2 gʱ�������������9.2 g������У�NaHCO3��KHCO3�����ʵ����ֱ�Ϊn(NaHCO3)��n(KHCO3)�����У�n(NaHCO3)��n(KHCO3)��0.1 mol,84 g��mol��1 ��n(NaHCO3)��100 g��mol��1��n(KHCO3)��9.2 g��������ʽ��ã�n(NaHCO3)��n(KHCO3)��0.05 mol��C����ȷ��NaHCO3����������С��KHCO3������������B���ȷ������������ʱ������3.36 L�����������������Ϊ9.2 g��1.5��13.8 g��D���ȷ��

 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д����ֺ��ؽ��У�����ˮ��ȫռ�м�Ϊ��Ҫ�ĵ�λ�� ij�о�С����ȡ��������Ⱦ��ˮԴ���з�����������������ʵ����Ϣ������һ������Ⱦ��ˮԴ����A��B�������ʣ�һ������C��D�������ʣ�һ������E���ʣ�A��B��C��D��E���ֳ��������ﶼ�����±��е������γɵģ�
| ������ | K����Na����Cu2����Al3�� |
| ������ | SO |
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�У����ְ�ɫ�����������μӳ����ܽ⡣
�۽�����ɫ��Ӧʵ�飬ֻ��B��C���м����ӡ�
���ڸ���Һ�м���Ba(NO3)2��Һ���ټ������ϡ���ᣬA�зų���ɫ���壬C��D�в�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɡ�
��������ʵ��������д���пհף�
(1)д��B��C��D�Ļ�ѧʽ��B________��C________��D________��
(2)����1 mol A����Һ�뺬1 mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ϊ________��
(3)д��ʵ��ڷ�����Ӧ�����ӷ���ʽ________________________________________
________________________________________________________________________��
(4)C��������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��_______________________________
________________________________________________________________________��
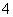 ��5H2O2��6H��===2Mn2����8H2O��5O2��
��5H2O2��6H��===2Mn2����8H2O��5O2��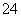 )��1.0��10��6 mol��L��1��Ӧ��
)��1.0��10��6 mol��L��1��Ӧ�� ����Һ��c(Ba2��)��________mol��L��1��
����Һ��c(Ba2��)��________mol��L��1�� ��������ͬ���ڷ�����
��������ͬ���ڷ�����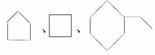 ͬ���ڻ�����
ͬ���ڻ�����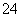 ��HCO
��HCO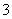 ��NO
��NO