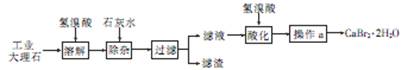
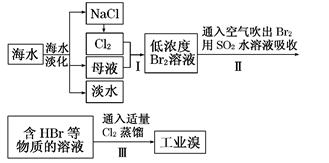
��Ŀ����
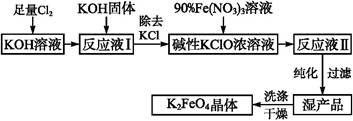
��ҵ�����������ᣨ�е㣺90��C��ʱ��ͬʱ�������������ƣ��乤���������£�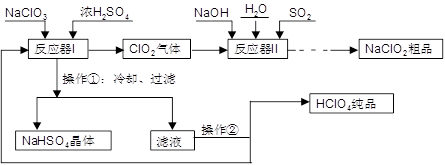
��1��ʵ���ҽ��й��˲����ij��ò��������� ��
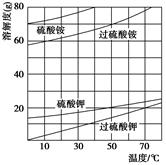
��2����Ӧ��I�е��¶����Ϊ ������ţ��������ڵ�����Ϊ ��
A. 0��C �� B. 20��C �� C. 80��C �� D. 120��C
��3����Ӧ��II�з�����Ӧ�����ӷ���ʽΪ ��
��4���ӿ췴Ӧ��II�з�Ӧ���ʵĴ�ʩ�� ��д��һ�ִ�ʩ���ɣ��ȡ��ӷ�Ӧ��II�л��NaClO2 ��Ʒ��ʵ����������� ������ţ���ͬ������һ���ᴿ�IJ�������Ϊ ��
A������ B���ؽᾧ C������ D������Ũ�� E���������� F����ȴ�ᾧ G����ȡ��Һ
��5�����������п�ѭ��ʹ�õ�����Ϊ ������Ʒ��NaClO2��NaHSO4��� ���ѧʽ����
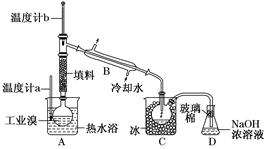
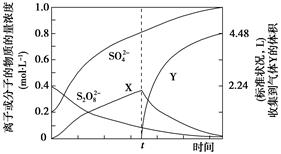
��1������ͨ��©�������������ձ���3�֣���1�֣�
��2��C�� ���� ����1�֣�
��3��2ClO2+SO2+4OH-=2ClO2-+SO42-+2H2O ��2�֣�
��4���ʵ������¶ȣ�����NaOH��Һ��Ũ�ȡ��������壨SO2��ClO2����NaOH��Һ�ĽӴ�����ȣ���2�֣���DF(A)��2�֣���B��1�֣�
��5�����Na2SO4����2�֣�
������������� ��1��ʵ���ҳ��õIJ��������У���ͨ��©�������������ձ��������ʱ������д��ȫ������������ʱ����ʵ��ʱ���龳�����������벻���׳�����
��2����Ӧ��I�м������������Ũ����������·�����������ԭ��Ӧ��Cl���ϼ�+4��+5��+7����˵õ��ĺ��Ȼ���������ClO2 ��������ᣬ����Ҫ�õ��ϸ�Ũ�ȵĸ�������Һ��Ӧ�þ����ܵ�����ʹClO2 ���������ͬʱ�¶Ȳ��ܹ��ߣ�����������е��¶�90��C����ʹ��������������������¶�Ӧ��ѡC��80��C�����ں�һ�ε���Һ���ٻ�ô����ĸ������Ʒ������ѡ�����������¶���������е��¶ȣ�����õ������ᴿƷ��
��3���ӷ�ӦII��ǰ��Ӧ��Ͳ�����Կ�����Cl�Ļ��ϼ�+4��+3��������������ԭ��Ӧ��ClO2 �������������� SO2�ڷ�Ӧ�б�����Ϊ��SO42-���м���뷴Ӧ�����κ�ˮ�����Ը���������ԭ��ʧ�����غ����ȱ����ƽ���ɵ÷�Ӧ�����ӷ���ʽΪ2ClO2+SO2+4OH-=2ClO2-+SO42-+2H2O����4��Ӱ�췴��ѧӦ���ʵ��������¶ȡ�Ũ�ȡ��Ӵ�����ȣ������ڷ�Ӧ��II��Ҫ�ӿ췴Ӧ���ʣ�����ͨ���ʵ������¶ȡ��������������Ƶ�Ũ�ȡ���������Һ�ĽӴ�����ȴ�ʩ���ı䡣��Ҫ�Ӵӷ�Ӧ��II�л��NaClO2 �����Ʒ������ͨ������Ũ������ȴ�ᾧ�����˵ķ����õ�����ѡDFA����һ���ᴿ��Ʒ���壬��Ӧ�öԸôֲ�Ʒ�����ؽᾧ������
��5���������̷�Ϊ2����������������������Ļ����У�����õ������ᴿƷʣ�µ���ҺΪ������Һ������������Һ����ѭ������Ӧ��I�м������ã�������������ƷNaClO2�л���������Na2SO4�����Ը���Ʒ����NaClO2��NaHSO4��Na2SO4 ��
���㣺���⿼����ǻ�ѧ���������⣬��Ƽ�����ʵ�����ݡ�������ԭ��Ӧ����ʽ��ƽ�����ݡ�

 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
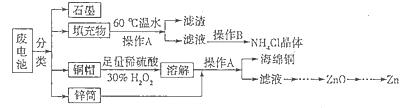

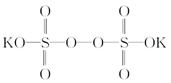 ������ǿ�����ԣ�������ԭΪ����أ���80 �����������ֽ⡣ʵ����ģ�ҵ�ϳɹ�����ص��������£�
������ǿ�����ԣ�������ԭΪ����أ���80 �����������ֽ⡣ʵ����ģ�ҵ�ϳɹ�����ص��������£�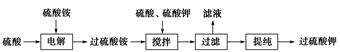
 KCl+KClO+H2O(����:�¶Ƚϵ�)
KCl+KClO+H2O(����:�¶Ƚϵ�) ;��������������������
;��������������������