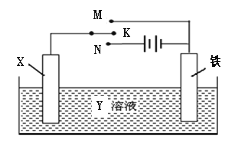
��Ŀ����
����Ŀ��ij50 mL��Һ�п��ܺ���H+��Na+��Mg2+��Al3+��SO42-��NH4+���ӣ��������Һ�м���5 mol/L��NaOH��Һʱ���������ɳ��������ʵ���n(����)��NaOH��Һ�����v(NaOH)�仯��ϵ����ͼ��ʾ��
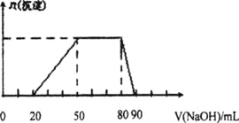
A��ԭ��Һ��һ�����е���������H+��Mg2+��Al3+��SO42-
B�� ԭ��Һ��Al3+��Ũ��Ϊlmol/L
C�� ԭ��Һ��NH4+�����ʵ���Ϊ0.4 mol
D�� �������NaOH��Һ�����Ϊ90mLʱ����Ӧ����Һ�е�����ֻ��Na+��SO42-
���𰸡�B
��������
�����������ͼ��֪����ʼ��������кͣ�Ȼ��Al3+��Ӧ����NH4+��Ӧ����������Ӧ��ȫ�ܽ⣬������Һ�к��е������У�H+��Al3+��NH4+�����ݵ����Կ�֪һ���������������һ������������ΪMg2+��Na+����ȷ������A��ԭ��Һ��һ�����е���������H+��NH4+��Al3+��A����B������ͼ���֪�ܽ�����������������������Һ��10mL���������Ƶ����ʵ�����0.05mol������ԭ��Һ��Al3+��Ũ��Ϊ0.05mol��0.05L��lmol/L��B��ȷ��C��笠���������������Һ�������30mL�����ʵ�����0.15mol�����ԭ��Һ��NH4+�����ʵ���Ϊ0.15mol��C����D���������NaOH��Һ�����Ϊ90mLʱ����Ӧ����Һ�е�������Na+��SO42-��������ƽ��������ӣ�D����ѡB��

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�