��Ŀ����
�ü״��������а��ķ�ˮ����ij��ϸ���������£��������·�Ӧ��
��NH3��O2 HNO3��H2O
HNO3��H2O
��6HNO3��5CH3OH 3N2����5CO2����13H2O
3N2����5CO2����13H2O
(1)����ʽ����ƽ��Ļ�ѧ�������ֱ�Ϊ��________��
(2)��ij��ˮ����ʱ������95��ת��Ϊ���ᣬ������84��ת��Ϊ����������ˮ�к���34mg/L�����������ķ�ˮ500m3��������״�������(kg)�ͷų����������(L)��
�𰸣�1��2��1��1;21.28kg��8937.6L
������
������

��ϰ��ϵ�д�
�����Ŀ
�����ű�����ȫ����̡�ʡ��֮�Ƶĸ�����������ȴƵƵ�ܵ���������ţ�������ˮԴ��ˮ��״�����棬����9�������е�34������ʽ��������ˮԴ��ˮ�ʴ����Ϊ73.3%��������ͬ�������½������Ա������������ǿ̲��ݻ������Σ�
�����������ǿ��ƶ���������Ⱦ����Ҫ�����ֶΣ�
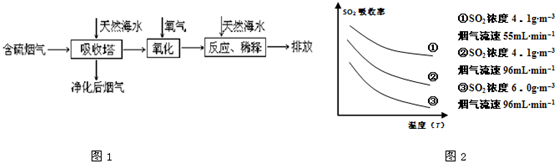
��1�����ú�ˮ������һ����Ч�ķ������乤��������ͼ1��ʾ��
ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ����������Ȼ��ˮ��pH��8�����պ���������ģ��ʵ�飬ʵ������ͼ2��ʾ��
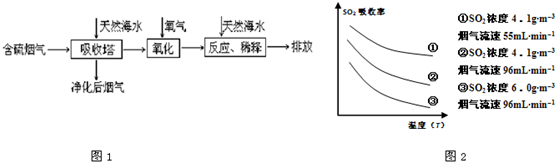
�ٸ���ͼʾʵ������Ϊ�����һ��Ũ�Ⱥ���������SO2������Ч�ʣ����д�ʩ��ȷ����______��������ĸ��ţ�
A������ͨ�뺬���������¶ȡ���������B����Сͨ�뺬������������
C��������Ȼ��ˮ�Ľ����������������� D������Ȼ��ˮ�м�����ʯ��
����Ȼ��ˮ�����˺��������������H2SO3��ʹ�ÿ����е���������������д���÷�Ӧ�Ļ�ѧ����ʽ��______��������ġ���ˮ����Ҫ�ô�������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ����______��
��2��ʯ��ʯ-ʯ��ʪ�����������ռ����Ĺ���ԭ���������еĶ��������뽬Һ�е�̼����Լ�����Ŀ�����Ӧ����ʯ�ࣨCaSO4?2H2O����д���÷�Ӧ�Ļ�ѧ����ʽ��______��
���ؽ������ӶԺ������������������Ⱦ��ij��������ˮ��pH��2���к���Ag+��Pb2+���ؽ������ӣ���Ũ�ȸ�ԼΪ0.01mol?L-1���ŷ�ǰ���ó�������ȥ���������ӣ������й��������£�
| ���ܵ���� | AgI | AgOH | Ag2S | PbI2 | Pb��OH��2 | PbS |
| Ksp | 8.3��10-17 | 5.6��10-8 | 6.3��10-50 | 7.1��10-9 | 1.2��10-15 | 3.4��10-28 |
A��NaOH����B��Na2S����C��KI�� D��Ca��OH��2
��4�����ֻ����ʯ�Ҵ�������Pb2+�ķ�ˮ��ʹ��Һ��pH=8.0��������ķ�ˮ�У�c��Pb2+��=______��������Ҫ����ˮ�ۺ��ŷű�Ϊc��Pb2+������1.0��l0-8mol?L-1���ʴ�����ķ�ˮ�Ƿ�����ŷű���______ ����ǡ�����
�����ű�����ȫ����̡�ʡ��֮�Ƶĸ�����������ȴƵƵ�ܵ���������ţ�������ˮԴ��ˮ��״�����棬����9�������е�34������ʽ��������ˮԴ��ˮ�ʴ����Ϊ73.3%��������ͬ�������½������Ա������������ǿ̲��ݻ������Σ�
�����������ǿ��ƶ���������Ⱦ����Ҫ�����ֶΣ�
��1�����ú�ˮ������һ����Ч�ķ������乤��������ͼ1��ʾ��
ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ����������Ȼ��ˮ��pH��8�����պ���������ģ��ʵ�飬ʵ������ͼ2��ʾ��
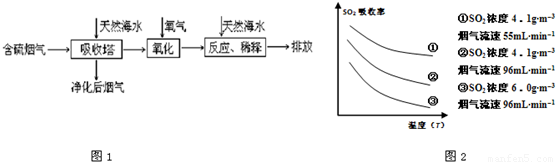
�ٸ���ͼʾʵ������Ϊ�����һ��Ũ�Ⱥ���������SO2������Ч�ʣ����д�ʩ��ȷ����______��������ĸ��ţ�
A������ͨ�뺬���������¶� B����Сͨ�뺬������������
C��������Ȼ��ˮ�Ľ����� D������Ȼ��ˮ�м�����ʯ��
����Ȼ��ˮ�����˺��������������H2SO3��ʹ�ÿ����е���������������д���÷�Ӧ�Ļ�ѧ����ʽ��______��������ġ���ˮ����Ҫ�ô�������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ����______��
��2��ʯ��ʯ-ʯ��ʪ�����������ռ����Ĺ���ԭ���������еĶ��������뽬Һ�е�̼����Լ�����Ŀ�����Ӧ����ʯ�࣮д���÷�Ӧ�Ļ�ѧ����ʽ��______��
���ؽ������ӶԺ������������������Ⱦ��ij��������ˮ��pH��2���к���Ag+��Pb2+���ؽ������ӣ���Ũ�ȸ�ԼΪ0.01mol?L-1���ŷ�ǰ���ó�������ȥ���������ӣ������й��������£�
��3������Ϊ����ˮ��Ͷ��______������ĸ��ţ�������Ч����ã�
A��NaOH B��Na2S C��KI D��Ca��OH��2
��4�����ֻ����ʯ�Ҵ�������Pb2+�ķ�ˮ��ʹ��Һ��pH=8.0��������ķ�ˮ�У�c��Pb2+��=______��������Ҫ����ˮ�ۺ��ŷű�Ϊc��Pb2+������1.0×l0-8mol?L-1���ʴ�����ķ�ˮ�Ƿ�����ŷű���______ ����ǡ�����
�����������ǿ��ƶ���������Ⱦ����Ҫ�����ֶΣ�
��1�����ú�ˮ������һ����Ч�ķ������乤��������ͼ1��ʾ��
ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ����������Ȼ��ˮ��pH��8�����պ���������ģ��ʵ�飬ʵ������ͼ2��ʾ��

�ٸ���ͼʾʵ������Ϊ�����һ��Ũ�Ⱥ���������SO2������Ч�ʣ����д�ʩ��ȷ����______��������ĸ��ţ�
A������ͨ�뺬���������¶� B����Сͨ�뺬������������
C��������Ȼ��ˮ�Ľ����� D������Ȼ��ˮ�м�����ʯ��
����Ȼ��ˮ�����˺��������������H2SO3��ʹ�ÿ����е���������������д���÷�Ӧ�Ļ�ѧ����ʽ��______��������ġ���ˮ����Ҫ�ô�������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ����______��
��2��ʯ��ʯ-ʯ��ʪ�����������ռ����Ĺ���ԭ���������еĶ��������뽬Һ�е�̼����Լ�����Ŀ�����Ӧ����ʯ�࣮д���÷�Ӧ�Ļ�ѧ����ʽ��______��
���ؽ������ӶԺ������������������Ⱦ��ij��������ˮ��pH��2���к���Ag+��Pb2+���ؽ������ӣ���Ũ�ȸ�ԼΪ0.01mol?L-1���ŷ�ǰ���ó�������ȥ���������ӣ������й��������£�
| ���ܵ���� | AgI | AgOH | Ag2S | PbI2 | Pb��OH��2 | PbS |
| Ksp | 8.3×10-17 | 5.6×10-8 | 6.3×10-50 | 7.1×10-9 | 1.2×10-15 | 3.4×10-28 |
A��NaOH B��Na2S C��KI D��Ca��OH��2
��4�����ֻ����ʯ�Ҵ�������Pb2+�ķ�ˮ��ʹ��Һ��pH=8.0��������ķ�ˮ�У�c��Pb2+��=______��������Ҫ����ˮ�ۺ��ŷű�Ϊc��Pb2+������1.0×l0-8mol?L-1���ʴ�����ķ�ˮ�Ƿ�����ŷű���______ ����ǡ�����