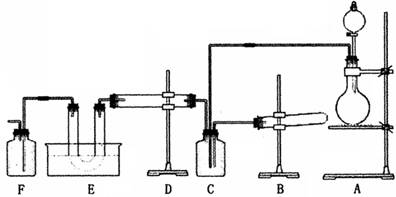
��Ŀ����
(12��)(1)������ش�ijͬѧ��̽��Ũ���ᡢϡ���ᡢŨ���ᡢϡ�������������������ǿ����ʵ���з������й����⣺
�ֱ���ʢ�е���ͭƬ����֧�Թ��м��������Ģ�Ũ�����ϡ�����Ũ�����ϡ���ᣬ���漴������Ӧ����(����ţ���ͬ)________����������Ӧ����________�����Ⱥ�����Ӧ����________������Ҳ��������Ӧ����________���ɴ˿��Եõ�������������������ǿ������˳����________��
(2)���ᡢ��������ѧ�γ������ᡣ����������ͭ��Ӧ��������ش��������⣺
������֪����ϡ������ͭ����Ӧ������ϡ�����м���H2O2�����ʹͭ�ܽ⡣�÷�Ӧ�Ļ�ѧ����ʽΪ
________________________________________________________________________��
����һ�������18 mol��L��1��Ũ�����м��������ͭƬ������ʹ֮��Ӧ������ԭ��������0.9 mol����������ʵ�����________(����ڡ������ڡ���С�ڡ�)100 mL����ͬѧ�������ʹʣ���ͭƬ�����ܽ⣬�������м��������Σ�������________(����С������С�)��
�ֱ���ʢ�е���ͭƬ����֧�Թ��м��������Ģ�Ũ�����ϡ�����Ũ�����ϡ���ᣬ���漴������Ӧ����(����ţ���ͬ)________����������Ӧ����________�����Ⱥ�����Ӧ����________������Ҳ��������Ӧ����________���ɴ˿��Եõ�������������������ǿ������˳����________��
(2)���ᡢ��������ѧ�γ������ᡣ����������ͭ��Ӧ��������ش��������⣺
������֪����ϡ������ͭ����Ӧ������ϡ�����м���H2O2�����ʹͭ�ܽ⡣�÷�Ӧ�Ļ�ѧ����ʽΪ
________________________________________________________________________��
����һ�������18 mol��L��1��Ũ�����м��������ͭƬ������ʹ֮��Ӧ������ԭ��������0.9 mol����������ʵ�����________(����ڡ������ڡ���С�ڡ�)100 mL����ͬѧ�������ʹʣ���ͭƬ�����ܽ⣬�������м��������Σ�������________(����С������С�)��
(1)�ۡ��ܡ��١��ڡ���>��>��>��
(2)��Cu��H2O2��2HCl===CuCl2��2H2O
�ڴ��ڡ�����?
(2)��Cu��H2O2��2HCl===CuCl2��2H2O
�ڴ��ڡ�����?
(1)�Ƚϲ�ͬ����������ǿ��ʱ��������ͬһ�ֻ�ԭ�������Ƿ�Ӧʱ��Ӧ�����������бȽϡ�Cu��Ũ�����ڳ����¾��ҷ�Ӧ��Cu��ϡ������Ȼ���ʱ���ɷ�Ӧ��Cu��Ũ�������ڼ��������²��ܷ�Ӧ��Cu��ϡ�������Ҳ����Ӧ��(2)H2O2���н�ǿ�����ԣ������������¿���������ͭ�������������£������ξ���ǿ�����ԣ���ʹCu��Ũ���ᷴӦ��ʣ���ͭƬ�����ܽ⡣

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д�
�����Ŀ
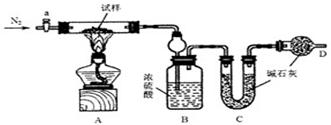

 ����
����