��Ŀ����
4��������K3Fe��A2B4��3•3H2O����Ҫ�Ĵ�������������K3Fe��A2B4��3•3H2O������ȫ�ֽ⣬ֻ�õ����������������������������ֻ�мס��Һ�ˮ��������֪�ס��Ҿ���A��B��Ԫ����ɣ���Ħ��������M���ף���M���ң�
��AԪ�ص������������Ǵ���������2����BԪ�ص�������������������������3����
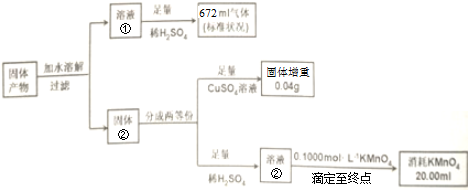
���������������ֻ��Fe��FeO��K2AB3��ijͬѧ�ٽ������¶���������
��1���Ļ�ѧʽ��CO��
��2����Һ����KMnO4����������ԭ�����ӷ���ʽ��MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O��
��3��������ʵ�����ݵķ�����֪n��Fe����n��FeO����n��K2AB3��=1��1��3��
��4��ijͬѧ��Ϊ����Һ�����Ϻ�ɫ��Ϊ��ɫ��������������ڲ���ɫ������֤����Һ����KMnO4��Һ��Ӧ����ζ��յ㣮
�жϸ�ͬѧ����ĺ����Բ�˵�����ɲ�������ӦΪ�������һ�θ��������Һ����Һǡ������ɫ��Ϊ��ɫ��
���� AԪ�ص������������Ǵ���������2����AΪCԪ�أ�BԪ�ص�������������������������3����BΪOԪ�أ��ס��Ҿ���C��O��Ԫ����ɣ���Ħ��������M���ף���M���ң����ʼ�ΪCO����ΪCO2��������K3Fe��C2O4��3•3H2O������ȫ�ֽⷽ��ʽΪ2K3Fe��C2O4��3•3H2O=3K2CO3+Fe+FeO+5CO2+4CO+6H2O���������ֻ��Fe��FeO��K2CO3����ˮ�ܽ����Һ��Ϊ̼��أ�����ΪFe��FeO������������ͭ��Һ��Ӧ����ͭ���ʣ�Fe��FeO���������ᷴӦ����Ӧ���ɵĶ���������KMnO4��Һ�ζ���
��� �⣺AԪ�ص������������Ǵ���������2����AΪCԪ�أ�BԪ�ص�������������������������3����BΪOԪ�أ��ס��Ҿ���C��O��Ԫ����ɣ���Ħ��������M���ף���M���ң����ʼ�ΪCO����ΪCO2��������K3Fe��C2O4��3•3H2O������ȫ�ֽⷽ��ʽΪ2K3Fe��C2O4��3•3H2O=3K2CO3+Fe+FeO+5CO2+4CO+6H2O���������ֻ��Fe��FeO��K2CO3����ˮ�ܽ����Һ��Ϊ̼��أ�����ΪFe��FeO������������ͭ��Һ��Ӧ����ͭ���ʣ�Fe��FeO���������ᷴӦ����Ӧ���ɵ�����������ʹKMnO4��Һ��ɫ��
��1���Ļ�ѧʽ��CO���ʴ�Ϊ��CO��
��2����Һ��Ϊ������������KMnO4����������ԭ�����ӷ���ʽ��MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O���ʴ�Ϊ��MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O��
��3��������K3Fe��C2O4��3•3H2O������ȫ�ֽⷽ��ʽΪ2K3Fe��C2O4��3•3H2O=3K2CO3+Fe+FeO+5CO2+4CO+6H2O���ʴ�Ϊ��1��1��3��
��4�����������Һ����ɫ��������������������Һǡ�÷�Ӧʱ����Һ����ɫ�ģ���������ع���ʱ��Һ����ɫ���ø��������Һ�ζ�����������Һ���յ㣺�������һ�θ��������Һ����Һǡ������ɫ��Ϊ��ɫ���ʴ�Ϊ����������ӦΪ�������һ�θ��������Һ����Һǡ������ɫ��Ϊ��ɫ��
���� ���⿼��̽�����ʵ���ɻ�������ʵĺ�����Ϊ��Ƶ���㣬�������ʵ��ƶ�Ϊ���Ĺؼ�������������ԭ��Ӧ��ƽ����������Ŀ��飬��Ŀ�Ѷ��еȣ�

�ٽ�35.5g Na2SO4����250mLˮ��
�ڽ�80.5g Na2SO4•10H2O��������ˮ�У�����ˮϡ����250mL
�۽�50mL 5.0mol/L Na2SO4��Һ��ˮϡ����250mL��
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �� |
| A�� | ����ϩ���ϵ��ϻ������ڷ����˼ӳɷ�Ӧ | |
| B�� | ú����������Һ���������仯����ת��Ϊ���ȼ�� | |
| C�� | ���ڳ�ʪ�Ŀ����з��ã�������ѧ��ʴ������ | |
| D�� | �����ѧ�Ҹ���ڹ��˴�����Ϣ������ȡ��ͻ���Գɾͣ����˵���Ҫ�ɷ��Ǹߴ��ȵĶ������� |
| A�� | �����£���Ӧ2A ��s��+B ��g���T2C ��g��+D ��g�������Է����У���÷�Ӧ��Hһ������0 | |
| B�� | 101kPaʱ��2 H2��g��+O2��g���T2H2O��l����H=-571.6kJ•mol-1����H2��ȼ����Ϊ571.6kJ•mol-1 | |
| C�� | C ��ʯī��s���TC �����ʯ��s����H1=+1.9kJ•mol-1������ʯī��ȡ���ʯ�����ȷ�Ӧ�����ʯ��ʯī�ȶ� | |
| D�� | 0.5molH2SO4��0.5mol Ba��OH��2��ȫ��Ӧ���ų���������Ϊ�к��� |
��Ca��OH��2����BaCl2����Na2CO3����Na2SiO3����Ba��NO3��2���ޱ����ƣ�
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �ܢݢ� | D�� | �ۢݢ� |
| A�� | Al3+�Ľṹʾ��ͼ��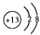 | B�� | ˮ�ĵ���ʽ��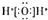 | ||
| C�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | D�� | ����7�����ӵ�̼ԭ�ӣ�${\;}_{6}^{7}$C |
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� |
��2���������ڵ�Ԫ���γɵĵ�ԭ�����Ӱ뾶������P3-���ѧʽ����
��3���ٺ͢ڰ�������3��8�γɵĻ�����A�ĵ���ʽΪ
 ��������Aͨ���ɢں͢��γɵ������ӵ�����Һ�����ӷ���ʽΪAlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
��������Aͨ���ɢں͢��γɵ������ӵ�����Һ�����ӷ���ʽΪAlO2-+CO2+2H2O=Al��OH��3��+HCO3-����4���õ���ʽ��ʾԪ�آ���ߵĻ�������γɹ��̣�
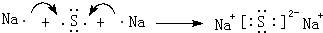 ��
�� | A�� | N2 | B�� | SO2 | C�� | CO2 | D�� | CO |