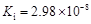��Ŀ����
�����£���H2O2��Һ�еμ�����FeSO4��Һ���ɷ�������������Ӧ��
��2Fe2++H2O2+2H+��2Fe3++2H2O�� ��2Fe3++H2O2��2Fe2++O2��+2H+������˵������ȷ���� �� ��
A��H2O2�������Ա�Fe3+ǿ���仹ԭ�Ա�Fe2+ǿ
B����H2O2�ֽ�����У���Һ��pH���½�
C����H2O2�ֽ�����У� Fe2+��Fe3+���������ֲ���
D��H2O2��������Ҫ�ϸ�������Fe2+
B
����:
��������ԭ��Ӧ��ǿ���ɿ�֪�����������������������������ԭ���ǻ�ԭ�����ڻ�ԭ����ɷ���ʽ�ٿ�֪��������H2O2�����Ա���������Fe3+ǿ���ɷ���ʽ�ڿ�֪����ԭ��H2O2��ԭ�Ա�ԭ����ΪFe2+ǿ������A��ȷ������ʽ�ٺͷ���ʽ����ӣ���H2O2�ֽ�����H2O��O2��H2O2�������ԣ��������ŷ�Ӧ���У�pH���ߣ�B����H2O2�ֽ�ʱFe3+��Fe2+�������������������䣬C��ȷ����ΪFe2+�ɵ���H2O2�ֽ⣬����H2O2��������Ҫ�������Fe2+��D��ȷ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���������ĵ���ƽ�ⳣ�����±���
|
���� |
HCOOH |
HClO |
H2CO3 |
H2SO3 |
|
����ƽ�ⳣ�� ��25�棩 |
|
|
|
|
��1�����¶���ͬʱ���������Kiֵ�����Ե����ǿ���Ĺ�ϵΪ��________________________��
��2���������ӷ���ʽ��ȷ����
A��2ClO�� + H2O + CO2 �� 2HClO + CO32��
B��2HCOOH + CO32�� �� 2HCOO�� + H2O + CO2��
C��H2SO3 + 2HCOO�� �� 2HCOOH + SO32��
D��Cl2 + H2O+2CO32�� �� 2HCO3�� + Cl�� + ClO��
��3�������£�pH=3��HCOOH��Һ��pH=11��NaOH��Һ�������Ϻ���Һ������Ũ���ɴ�С��˳��Ϊ������������������������������������������������������������������
������(H2SeO3)Ҳ��һ�ֶ�Ԫ���ᣬ��������һ����ɫ���壬������ˮ���н�ǿ�������ԡ�
��4������������Һ�в���ͨ��SO2 ��������ɫ���ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ������������������������ ��������������������������������������������
��5������������30%��H2O2���ȿ��Ƶ�����(H2SeO4)����Ӧ����ʽ���£�
H2SeO3 + H2O2 �� H2SeO4+H2O������˵������ȷ���ǡ���������������������������������
A��H2O2�������������ǻ�ԭ��
B��H2O �Ȳ������������ֲ��ǻ�ԭ����
C��H2SeO4���������������ǻ�ԭ����
D�������ԣ�H2SeO3��H2SeO4
���ᣨH6TeO6����һ�ֺ������ᣬ �������������Ա����ỹҪǿ�������Խ����У�����ɽ�HI������I2������ʽ���£�
�������������Ա����ỹҪǿ�������Խ����У�����ɽ�HI������I2������ʽ���£�
���� ��HI+���� ��H6TeO6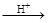 ���� ��TeO2+�� ����Te+���� ��I2+�� ����H2O
���� ��TeO2+�� ����Te+���� ��I2+�� ����H2O
��6������Ӧ�����ɵ�TeO2��Te�����ʵ���֮��Ϊ ������ƽ������ѧ����ʽ��
������ƽ������ѧ����ʽ��