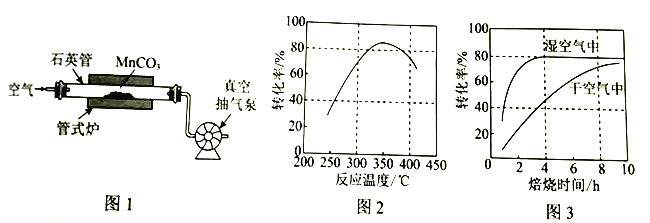
��Ŀ����
����Ŀ��ȡ3mL 5mol��L-1 H2O2��Һ����������MnO2��ĩ��2minʱc(H2O2)��Ϊ1mol��L-1������˵������ȷ����
A.2min�ڣ�v(H2O2) =2molL-lmin-1
B.MnO2�Ǵ������ܼӿ�H2O2�ķֽ�����
C.�����¶Ȼ�����H2O2��Ũ�ȣ����ܼӿ�H2O2�ķֽ�����
D.����5 molL-l H2O2��Һ���������6mL����H2O2�ķֽ���������ԭ����2��
���𰸡�D
��������
A. ��ѧ��Ӧ����v=![]() =
=![]() =2molL-lmin-1��A����ȷ��
=2molL-lmin-1��A����ȷ��
B. �����ı䷴Ӧ���ʲ��ı仯ѧƽ�⣬��������MnO2��ĩ�Ƿ�Ӧ�Ĵ������ܼӿ�H2O2�ķֽ����ʣ�B����ȷ��
C. �����¶Ȼ�����H2O2��Ũ�Ⱦ����Լӿ췴Ӧ���ʣ�C����ȷ��
D. ����5 molL-l H2O2��Һ���������6mL����ҺŨ�ȼ��٣�H2O2�ķֽ����ʻ������D�����
��ѡD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������Ľ������������ڸ���Ԫ�صľ������롣
��1��������һ���ʳ�������������� 6g��
����ȥ NaCl��Һ�е�SO42-�����μ������ҺΪ�������ʵĻ�ѧʽ��������______��Һ������______��Һ������ϡ���ᡣ
��ʳ�õ�����ʳ���м��� KIO3���±���ij���г������۵�һ�����ӵ�������װ�ϵIJ�������˵�����ش��������⣺
���ϱ� | ���ƺ��Ρ�����أ�KIO3�� |
������ | 20~40 mg/kg |
���ط��� | �ܷ�ܹ⡢���� |
ʳ�÷��� | ���ʱ��ʳƷ��������� |
ʳ�õ�������������ָ______��������������������Ԫ���������Ʋ������أ�KIO3����ѧ���ʣ�����أ�KIO3���ڸ���ʱ______�������ֽ����������ֽ�������һ�ֲⶨ�������Ļ�ѧԭ���ǣ�
������KIO3 + KI + H2SO4===K2SO4 + I2 + H2O��δ��ƽ����
������I2 + 2Na2S2O3 = 2NaI + Na2S4O8������Ӧ �� ��дΪ���ӷ���ʽ_____
��2������ÿ���ʳ������ȡ 0.8 g Ca Ԫ�ء�0.3 g Mg Ԫ�ء�
��Ca Ԫ���� Mg Ԫ�����ʵ���֮��Ϊ______��
��ī��Ƿ�(��Ҫ�ɷ��� CaCO3��������������θ����࣬��ԭ����_________(�����ӷ���ʽ�ش�)��
���Ӻ�ˮ�п���ȡþ����ȥ���� Mg(OH)2 �е� Ca(OH)2 ����ѷ����ǣ����������______������A������B������A. MgSO4 ��Һ��B. MgCl2 ��Һ�������ˣ�ϴ�ӣ����º�ɡ�