��Ŀ����
ij��Ԫ�ᣨ��ѧʽ��H2A��ʾ����ˮ�еĵ��뷽��ʽ�ǣ�
H2A==H++HA�� HA�� H++A2�� ����������⣺
H++A2�� ����������⣺
(1)Na2A��Һ��______������ԡ������ԡ����ԡ�����������________________�������ӷ���ʽ��ʾ����
(2)��֪0.1 mol��L��1��NaHA��ҺpH=2,��0.1 mol��L��1��H2A��Һ�������ӵ����ʵ���Ũ��_______0.11 mol��L-1(�������=����������
(3)��¯ˮ���е�CaSO4������Na2CO3��Һ������ת��ΪCaCO3�����������ȥ�������з�����CaSO4(1)+ CO32- CaCO3(1)+ SO42-����֪298Kʱ��Ksp[CaCO3]=2.80��10-9��Ksp[CaSO4]=4.90��10-5������¶��¸÷�Ӧ��ƽ�ⳣ��K= (������������λ��Ч����)
CaCO3(1)+ SO42-����֪298Kʱ��Ksp[CaCO3]=2.80��10-9��Ksp[CaSO4]=4.90��10-5������¶��¸÷�Ӧ��ƽ�ⳣ��K= (������������λ��Ч����)
H2A==H++HA�� HA��
 H++A2�� ����������⣺
H++A2�� ����������⣺(1)Na2A��Һ��______������ԡ������ԡ����ԡ�����������________________�������ӷ���ʽ��ʾ����
(2)��֪0.1 mol��L��1��NaHA��ҺpH=2,��0.1 mol��L��1��H2A��Һ�������ӵ����ʵ���Ũ��_______0.11 mol��L-1(�������=����������
(3)��¯ˮ���е�CaSO4������Na2CO3��Һ������ת��ΪCaCO3�����������ȥ�������з�����CaSO4(1)+ CO32-
 CaCO3(1)+ SO42-����֪298Kʱ��Ksp[CaCO3]=2.80��10-9��Ksp[CaSO4]=4.90��10-5������¶��¸÷�Ӧ��ƽ�ⳣ��K= (������������λ��Ч����)
CaCO3(1)+ SO42-����֪298Kʱ��Ksp[CaCO3]=2.80��10-9��Ksp[CaSO4]=4.90��10-5������¶��¸÷�Ӧ��ƽ�ⳣ��K= (������������λ��Ч����)(1) ���� B2��+H2O  HA��+OH�� (2) �� (3)1.75��10��4
HA��+OH�� (2) �� (3)1.75��10��4
 HA��+OH�� (2) �� (3)1.75��10��4
HA��+OH�� (2) �� (3)1.75��10��4�����������Ԫ�ᣨ��ѧʽ��H2A��ʾ����ˮ�еĵ��뷽��ʽ�ǣ�H2A==H++HA�� HA��
 H++A2�� ���ɴ˿���֪���ø���ĵ�һ��������ȫ�����ڶ������벻��ȫ��Ϊ���ᣬ��(1)Na2A��Һ�Լ��ԣ�������A���ӵ�ˮ�⣬�����ӷ���ʽΪ��B2��+H2O
H++A2�� ���ɴ˿���֪���ø���ĵ�һ��������ȫ�����ڶ������벻��ȫ��Ϊ���ᣬ��(1)Na2A��Һ�Լ��ԣ�������A���ӵ�ˮ�⣬�����ӷ���ʽΪ��B2��+H2O  HA��+OH����(2)��֪0.1 mol��L��1��NaHA��ҺpH=2,���� HA�������������Ũ��Ϊ0.01mol/L����H2A��Һ�������ӵ����ʵ���Ũ�����ڵ�һ�������ƶ�С��0.11 mol��L-1��(3)CaSO4(1)+ CO32-
HA��+OH����(2)��֪0.1 mol��L��1��NaHA��ҺpH=2,���� HA�������������Ũ��Ϊ0.01mol/L����H2A��Һ�������ӵ����ʵ���Ũ�����ڵ�һ�������ƶ�С��0.11 mol��L-1��(3)CaSO4(1)+ CO32- CaCO3(1)+ SO42-��ƽ�ⳣ��
CaCO3(1)+ SO42-��ƽ�ⳣ��K=c(SO42-)/c��CO32-)=Ksp[CaSO4]/Ksp[CaCO3]=4.90��10-5/=2.80��10-9=1.75��104��
���������⿼�����ε�ˮ�⡢�ܶȻ�������Ҫ�����Ԫ����ˮ�еĵ��뷽��ʽ�ǣ�H2A==H++HA��
HA��
 H++A2�� ���ɴ˿���֪���ø���ĵ�һ��������ȫ�����ڶ������벻��ȫ�������Ѷ��еȡ�
H++A2�� ���ɴ˿���֪���ø���ĵ�һ��������ȫ�����ڶ������벻��ȫ�������Ѷ��еȡ�
��ϰ��ϵ�д�
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
�����Ŀ


 M2+(aq)+2OH-(aq) Ksp=a��c(M2+)="b" mol��L-1ʱ����Һ��pH���ڣ� ��
M2+(aq)+2OH-(aq) Ksp=a��c(M2+)="b" mol��L-1ʱ����Һ��pH���ڣ� ��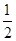 lg(
lg(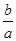 )
)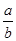 )
)