��Ŀ����
��������ͭ�Ƚ������仯�������ճ�������Ӧ�ù㷺�����������ʵ��ش����⣺
��1�������к���һ����̼������X(Fe3C)��X�������Ŀ����и������գ������д��ԵĹ���Y����Y���ڹ����������Һ�к��еĴ�����������________________��
��2��ij��Һ����Mg2����Fe2����Al3����Cu2�����������ӣ������м��������NaOH��Һ���ˣ��������������ղ������պ�Ĺ���Ͷ�뵽������ϡ�����У�������Һ��ԭ��Һ��ȣ���Һ�д������ٵ���������________��
A��Mg2�� B��Fe2�� C��Al3�� D��Cu2��
��3������������Ҫ��ҵ���ϣ��÷���м�Ʊ������������£�
��1�������к���һ����̼������X(Fe3C)��X�������Ŀ����и������գ������д��ԵĹ���Y����Y���ڹ����������Һ�к��еĴ�����������________________��
��2��ij��Һ����Mg2����Fe2����Al3����Cu2�����������ӣ������м��������NaOH��Һ���ˣ��������������ղ������պ�Ĺ���Ͷ�뵽������ϡ�����У�������Һ��ԭ��Һ��ȣ���Һ�д������ٵ���������________��
A��Mg2�� B��Fe2�� C��Al3�� D��Cu2��
��3������������Ҫ��ҵ���ϣ��÷���м�Ʊ������������£�
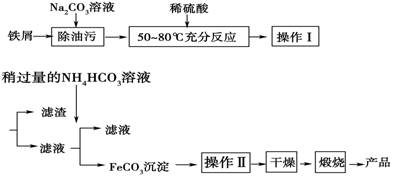
�ش��������⣺
�ٲ������������________��������ķ���Ϊ__________________________________��
��Na2CO3��Һ���Գ����ۣ�ԭ����(�����ӷ���ʽ��ʾ)______________________��
�����������FeCO3���������ӷ���ʽ____________________________��
��4����Щͬѧ��ΪKMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ(5Fe2����MnO4����8H��===5Fe3����Mn2����4H2O)��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����__________________________________��
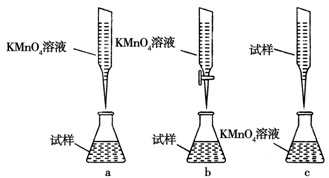
��ijͬѧ��Ƶ����еζ���ʽ�����������________��(�гֲ�����ȥ)(����ĸ���)
�ٲ������������________��������ķ���Ϊ__________________________________��
��Na2CO3��Һ���Գ����ۣ�ԭ����(�����ӷ���ʽ��ʾ)______________________��
�����������FeCO3���������ӷ���ʽ____________________________��
��4����Щͬѧ��ΪKMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ(5Fe2����MnO4����8H��===5Fe3����Mn2����4H2O)��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����__________________________________��
��ijͬѧ��Ƶ����еζ���ʽ�����������________��(�гֲ�����ȥ)(����ĸ���)
��1��Fe2����Fe3����H����
��2��BC
��3���ٹ��ˣ���©���м�����������ˮ����û��������������ˮ��Ȼ���£��ظ����Σ�
��CO32����H2O HCO3����OH������Fe2����2HCO3��===FeCO3����CO2����H2O
HCO3����OH������Fe2����2HCO3��===FeCO3����CO2����H2O
��4����250 mL����ƿ����b
��2��BC
��3���ٹ��ˣ���©���м�����������ˮ����û��������������ˮ��Ȼ���£��ظ����Σ�
��CO32����H2O
 HCO3����OH������Fe2����2HCO3��===FeCO3����CO2����H2O
HCO3����OH������Fe2����2HCO3��===FeCO3����CO2����H2O��4����250 mL����ƿ����b

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ








