��Ŀ����
��16�֣���������ͭ�Ƚ������仯�������ճ�����Ӧ�ù㷺�����������ʵ��ش����⣺
��1�������к���һ����̼������X��Fe3C���� X�������Ŀ����и������գ������д��ԵĹ���Y����Y���ڹ����������Һ�к��еĴ����������� ��X�����Ũ���ᷴӦ����Һ�к��е��εĻ�ѧʽ �����������Һ�к��е������ӵ��Լ�Ϊ________________
��2��ij��Һ����Mg2+��Fe2+��Al3+��Cu2+���������ӣ������м��������NaOH��Һ��,���ˣ��������������ղ������պ�Ĺ���Ͷ�������ϡ���ᣬ������Һ��ԭ��Һ��ȣ���Һ�д������ٵ���������________
A��Mg2+ B��Fe2+ C��Al3+ D��Cu2+
��3������������Ҫ�Ĺ�ҵ���ϣ��÷���м�Ʊ������������£�
�ش��������⣺
�ٲ������������ ��������������� ��
����������ķ���Ϊ_____________________________________________________________
_______________________________________________________________________________
��Na2CO3��Һ���Գ����ۣ�ԭ���ǣ������ӷ���ʽ��ʾ�� ��
�����������FeCO3���������ӷ���ʽ��Fe2+ + HCO3�� FeCO3��+ + H2O
��4����Щͬѧ��Ϊ��KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ��5Fe2++MnO4-+8H+===
5Fe3++Mn2++4H2O��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ��������
��ƽ�����������ձ�����ͷ�ι��⣬���� ��
�ڸ�ʵ���е�KMnO4��Һ��Ҫ�ữ�������ữ������ ��
a��ϡ���� b��ϡ���� c��ϡ���� d��Ũ����
��ijͬѧ��Ƶ����еζ���ʽ����������� ���гֲ�����ȥ��������ĸ��ţ�
��1��Fe2+ Fe3+ H+��Fe��NO3��3 KSCN��Һ
��2��BC ��
��3���ٹ��ˣ�ϴ�ӣ���©���м�����������ˮ,û������, ������ˮ��Ȼ����,�ظ�����.
�� CO32�� + H2O 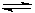 HCO3�� + OH��
HCO3�� + OH��
�� Fe2+ + 2HCO3- = FeCO3��+ CO2 �� + H2O��
��4����250mL����ƿ ��c ��b
����







