题目内容
【题目】已知常温时CH3COOH的电离平衡常数为K。该温度下向20mL0.1mol·L-1CH3COOH溶液中逐滴加入0.1mol·L-1NaOH溶液,其pH变化曲线如图所示(忽略温度变化)。下列说法中错误的是( )
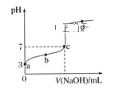
A.a点表示的溶液中c(CH3COO-)略小于10-3mol·L-1
B.b点表示的溶液中c(CH3COO-)>c(Na+)
C.c点表示CH3COOH和NaOH恰好反应完全
D.b、c点表示的溶液中![]() 均等于K
均等于K
【答案】C
【解析】
A、a点pH =3,说明c(H+) =1×10-3mol·L-1,,而氢离子浓度等于酸电离出的氢离子和水电离出的氢离子浓度之和,因此溶液中c(CH3COO-)略小于10-3mol·L-1,故A正确;
B、根据电荷守恒c(CH3COO-) +c(OH-) = c(H+) + c(Na+),b点溶液中c(OH-) < c(H+),因此b点溶液中c(CH3COO-) > c(Na+),故B正确;
C、c点是呈中性的点, CH3COOH和NaOH恰好反应完全时得醋酸钠溶液,溶液呈碱性,故C错误;
D、CH3COOH的电离平衡常数K=![]() ,K只与温度有关,与浓度无关,故D正确;
,K只与温度有关,与浓度无关,故D正确;
答案为C。

练习册系列答案
 名校练考卷期末冲刺卷系列答案
名校练考卷期末冲刺卷系列答案
相关题目