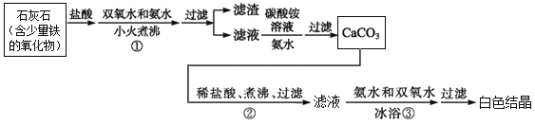
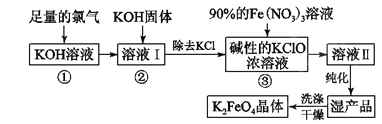
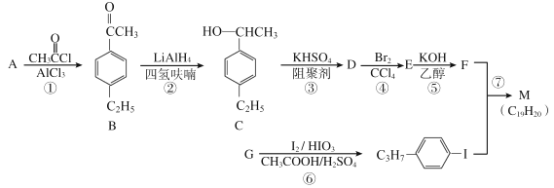
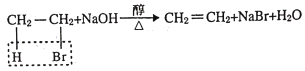
��Ŀ����
����Ŀ����������AlN����һ���������ǽ������ϣ�ijAlN��Ʒ������Al2O3���ʣ�Ϊ�ⶨAlN�ĺ����������������ʵ�鷽������֪��AlN+NaOH+H2O��NaAlO2��NH3��
������1��ȡһ��������Ʒ��������װ�òⶨ��Ʒ��AlN�Ĵ���(�г�װ������ȥ)��
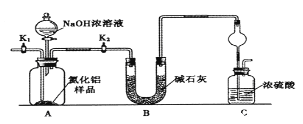
��1����ͼCװ�������θ���ܵ�������______________��
��2���������ʵ�鲽�裺��װ��ʵ��װ�ã�����______���ټ���ʵ��ҩƷ����������ʵ�������____,��Һ©������������NaOHŨ��Һ�������ٲ������塣��K1��ͨ�뵪��һ��ʱ�䣬�ⶨCװ�÷�Ӧǰ��������仯��ͨ�뵪����Ŀ����______________��
��3������װ�ô���ȱ�ݣ����²ⶨ���ƫ�ߣ�������Ľ����_________��
������2��������ͼװ�òⶨm g��Ʒ��A1N�Ĵ���(���ּг�װ������ȥ)��
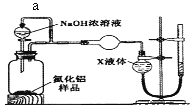
��4������a����Ҫ������______________��
��5��Ϊ�ⶨ������������������װ���е�XҺ�������____________������ѡ����ţ�
a��CCl4 b��H2O c��NH4Cl��Һ d��![]()
��6����mg��Ʒ��ȫ��Ӧ�����������������ΪVmL,����ת��Ϊ��״��������A1N����������Ϊ___���ú�V��m�Ĵ���ʽ��ʾ����
���𰸡���ֹ�������װ�������Թر�K1����K2��װ���в����İ���ȫ������Cװ��Cװ�ó��ڴ�����һ������װ�ñ�����ѹ�㶨��ʹNaOHŨ��Һ�������£�����������Һ������������������Ӱ��ad[41V/22400m]��100%
��������
��1����������Ũ�����ܷ�����Ӧ�����壬����������ͼCװ�������θ���ܵ������Ƿ�ֹ���������ã��ʴ�Ϊ����ֹ������
��2����װ��ʵ��װ�ã���Ҫ�ȼ��װ�������ԣ�����ʵ��ҩƷ����������ʵ������ǹر�K1����K2����Һ©������������NaOHŨ��Һ�������ٲ������壬��K1��ͨ�뵪��һ��ʱ�䣬�ⶨCװ�÷�Ӧǰ��������仯��ͨ�뵪����Ŀ���ǣ���Ӧ���ɰ���������װ���е�����ȫ������װ��C��Ũ�������գ�ȷ�ⶨװ��C�����ؼ��㣬�ʴ�Ϊ�����װ�������ԣ��ر�K1����K2����װ���в����İ���ȫ������Cװ�ã�
��3��װ�ô���ȱ���ǿ����е�ˮ�����Ͷ�����̼Ҳ���Խ���װ��C��ʹ�ⶨ���ƫ�ߣ���Ҫ����һ��ʢ��ʯ�Ҹ���ܣ��ʴ�Ϊ��Cװ�ó��ڴ�����һ������װ�ã�
��4������a���Ա��ַ�Һ©���Ϸ��ͷ�Ӧװ���ڵ���ѹ�㶨��ʹNaOHŨ��Һ�������£��ʴ�Ϊ��������ѹ�㶨��ʹNaOHŨ��Һ�������£�
��5��a��CCl4�����ܽⰱ���������������Ȼ�̼��Һ�ķ����ⶨ�����������a��ȷ�� b��������������ˮ��������ˮ���ⶨ����b���� c��������������ˮ����������NH4Cl��Һ�ķ����ⶨ�����������c���� d�����������ڱ������������ű���Һ���ⶨ�������������d��ȷ���ʴ�Ϊ��ad��
��6����m g��Ʒ��ȫ��Ӧ�����������������ΪV mL����ת��Ϊ��״������AlN+NaOH+H2O�TNaAlO2+NH3��
41 22.4L
m V��10-3L
m=![]() g����AlN����������=
g����AlN����������=![]() ��100%=
��100%=![]() ��100%���ʴ�Ϊ��
��100%���ʴ�Ϊ��![]() ��100%��
��100%��