��Ŀ����
(10��)��һ���̶�������ܱ���������2 mol A��1 mol B���������·�Ӧ��
2A(g) + B(g)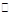 3C(g) + D(g)��2���Ӻ�Ӧ�ﵽƽ�⣬C��Ũ��Ϊ1.2 mol/L��
3C(g) + D(g)��2���Ӻ�Ӧ�ﵽƽ�⣬C��Ũ��Ϊ1.2 mol/L��
(1)��A��ʾ2������ƽ����Ӧ���� ��A�ڵ�1����ƽ������ ��2����ƽ�����ʣ��<������>������=������
(2)���¶����ߣ�ƽ��ʱ��������ƽ����Է���������С��������ӦΪ________������ȡ����ȡ�����Ӧ��
(3)��B��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_________���������գ�
�ټ���A �ڼ���B �ۼ�ѹ ���ټ���1.6 mol A+ 0.8 mol B �ݽ�C���������
(4)����ɱ䣬ά������ѹǿ���¶Ȳ��䣬�����з���������ʼ���ʣ��ﵽƽ��ʱC��Ũ����Ϊ1.2 mol/L����_________���������գ�
��4 mol A + 2 mol B ��3mol C + 1 mol D + l mol B
��3 mol C + 2 mol D ��1.6 mol A+ 0.8 mol B + 0.6 mol C + 0.2 mol D
2A(g) + B(g)
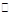 3C(g) + D(g)��2���Ӻ�Ӧ�ﵽƽ�⣬C��Ũ��Ϊ1.2 mol/L��
3C(g) + D(g)��2���Ӻ�Ӧ�ﵽƽ�⣬C��Ũ��Ϊ1.2 mol/L��(1)��A��ʾ2������ƽ����Ӧ���� ��A�ڵ�1����ƽ������ ��2����ƽ�����ʣ��<������>������=������
(2)���¶����ߣ�ƽ��ʱ��������ƽ����Է���������С��������ӦΪ________������ȡ����ȡ�����Ӧ��
(3)��B��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_________���������գ�
�ټ���A �ڼ���B �ۼ�ѹ ���ټ���1.6 mol A+ 0.8 mol B �ݽ�C���������
(4)����ɱ䣬ά������ѹǿ���¶Ȳ��䣬�����з���������ʼ���ʣ��ﵽƽ��ʱC��Ũ����Ϊ1.2 mol/L����_________���������գ�
��4 mol A + 2 mol B ��3mol C + 1 mol D + l mol B
��3 mol C + 2 mol D ��1.6 mol A+ 0.8 mol B + 0.6 mol C + 0.2 mol D
��10�֣�
��1��0.4 mol/(L��min) ��3�֣� > ��1�֣�
��2������ (2��)
��3���٢� (2��)
��4���٢� (2��)
��1��0.4 mol/(L��min) ��3�֣� > ��1�֣�
��2������ (2��)
��3���٢� (2��)
��4���٢� (2��)
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 pC(g)��qD(g) ��H��0�������������
pC(g)��qD(g) ��H��0�������������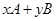

 �ﵽƽ�⣺
�ﵽƽ�⣺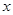 ��y��z��Ĺ�ϵ��________________��
��y��z��Ĺ�ϵ��________________�� ��������ѹǿʱ����ƽ�ⷢ���ƶ�����һ����________����������桱����Ӧ�����ƶ���
��������ѹǿʱ����ƽ�ⷢ���ƶ�����һ����________����������桱����Ӧ�����ƶ��� y C(g)����ͼ��ʾ�ڲ�ͬ�����·�Ӧ��B�����������(B)��ʱ��仯�Ĺ�ϵ���ݴ��ж�
y C(g)����ͼ��ʾ�ڲ�ͬ�����·�Ӧ��B�����������(B)��ʱ��仯�Ĺ�ϵ���ݴ��ж�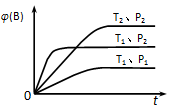
 Ur��(�������aq)��H��(aq)��(37��ʱ��Ka��4.0��10��6) ��NaUr(s)
Ur��(�������aq)��H��(aq)��(37��ʱ��Ka��4.0��10��6) ��NaUr(s)  Ur��(aq)��Na��(aq)
Ur��(aq)��Na��(aq) zC��g����ƽ��ʱ���A��Ũ��Ϊ0.50mol/L�������¶Ȳ��䣬���������ݻ�����ԭ������������
zC��g����ƽ��ʱ���A��Ũ��Ϊ0.50mol/L�������¶Ȳ��䣬���������ݻ�����ԭ������������ 2CH3OH��g������H=37kJ��mol-1����÷�Ӧ�ﵽƽ��ʱ���й��������£�
2CH3OH��g������H=37kJ��mol-1����÷�Ӧ�ﵽƽ��ʱ���й��������£�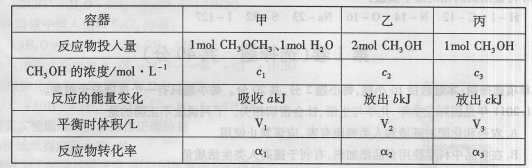

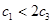
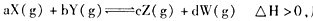 ��Ӧ5min�ﵽƽ��ʱ��X��Сn mol��L-1����Y��ʾ�ķ�Ӧ����Ϊ0.6n mol��L-1��min-1��������ϵѹǿ����W�İٷֺ����������仯��������������ȷ���� �� ��
��Ӧ5min�ﵽƽ��ʱ��X��Сn mol��L-1����Y��ʾ�ķ�Ӧ����Ϊ0.6n mol��L-1��min-1��������ϵѹǿ����W�İٷֺ����������仯��������������ȷ���� �� �� 2NH3 ��H��0����ʹ�ϳɰ���Ӧ���г̶�����ķ�����
2NH3 ��H��0����ʹ�ϳɰ���Ӧ���г̶�����ķ�����