��Ŀ����
3����1����ȥ����NaCl��Һ������NaHCO3���ʵ��Լ������ᣬ���ӷ���ʽΪHCO3-+H+=CO2��+H2O����2����ȥNa2CO3��ĩ�л����NaHCO3�����ü��ȷ�������ѧ����ʽΪ2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O��
��3����ȥMg���л��е�����Al���ʵ��Լ�������������Һ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��4����ȥFe2O3�л��е�Al2O3���ʵ��Լ�������������Һ�����ӷ���ʽΪAl2O3+2OH-�T2AlO2-+H2O��
���� ��1��NaHCO3�����ᷴӦ������NaCl��
��2��NaHCO3��NaOH��Ӧ����Na2CO3��
��3����������������Һ��Ӧ��
��4��������������������Һ��Ӧ��
��� �⣺��1����NaCl���ܺ����ᷴӦ��NaHCO3�������ᷴӦ��Ӧ�����Ȼ��ơ�ˮ��������̼�����ɵ��Ȼ���������ˮ�����Կ��������ȥ����NaCl��Һ������NaHCO3���ʣ����ӷ���ʽΪHCO3-+H+=CO2��+H2O���ʴ�Ϊ�����HCO3-+H+=CO2��+H2O��
��2����Na2CO3���Ȳ��ֽ⣬NaHCO3���ȷֽ�õ�̼���ơ�ˮ��������̼�����Կ��ü��ȵķ�����ȥNa2CO3��ĩ�л����NaHCO3���ʣ�����ʽΪ2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O���ʴ�Ϊ�����ȣ�2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O��
��3��Mg��Al���ǽ����������ᷴӦ������������������Һ��Ӧ��þ������������Һ����Ӧ�����Կ���ѡȡ����������Һ�����Ӽ������ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
�ʴ�Ϊ������������Һ��2Al+2OH-+2H2O=2AlO2-+3H2����
��4��������Ϊ�������������ǿ�Ӧ����Ӧ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O���ʴ�Ϊ������������Һ��Al2O3+2OH-�T2AlO2-+H2O��
���� ���������ʵ�����Ϊ���忼�������ʵļ���ͳ��Ӻͻ�ѧ��Ӧ����ʽ����д����ȷ���ʵ����ʲ�ע�������뷴Ӧ�Ĺ�ϵ�����ӵ�ԭ���ǽ����Ĺؼ�����Ŀ�ѶȲ���

| A�� | ZΪ0.3 mol•L-1 | B�� | X2Ϊ0.2 mol•L-1 | ||
| C�� | Y2Ϊ0.4 mol•L-1 | D�� | c��X2��+c��Y2��+c��Z��=0.55 mol•L-1 |
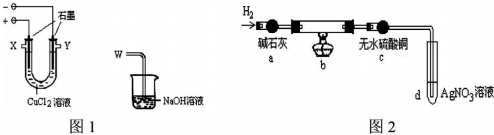
��1��ͼl�����ķ�Ӧʽ�ǣ�2Cl--2e-=Cl2����������W��Ӧ�������һX���ӣ�
��2��ʵ���������̼���ϳ��˸����к�ɫ���ʣ���������������ɫ���ʣ�ij��ѧ��ȤС��������Ͽ�֪��
| �������Ƽ���ѧʽ | �Ȼ���ͭCuCl | ��ʽ�Ȼ�ͭCu2��OH��3Cl |
| ���� | ��ɫ���塢����ˮ | ��ɫ���塢����ˮ |
�ٺ�ɫ���ʿ�����Cu��Cu2O������߶��У�
�ڰ�ɫ����ΪCuCl
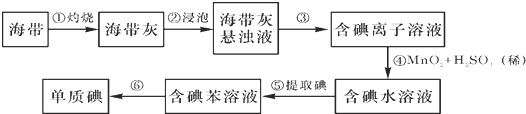
��3��Ϊ̽������̼���ϸ��ŵĺ�ɫ����ɫ���ʣ����������ʵ�飺ȡ������̼����ϴ�ӡ������������ΪW1g�����������ͼ2��ʾװ��b�У�����ʵ�飮ʵ���У�̼���ϵİ�ɫ������ȫ��Ϊ��ɫ����ˮ����ͭ����ɫ��d�г��ְ�ɫ������ʵ�����ʱ������ͨH2ֱ��̼����ȴ����������ΪW2g��
��̼���ϵĺ�ɫ������Cu����ˮ����ͭ�������Ǽ����ɫ����������Cu2O��
��d�з�Ӧ�����ӷ���ʽ��Ag++Cl-=AgCl����
��װ��b�з�����Ӧ�Ļ�ѧ����ʽ��2CuCl+H2$\frac{\underline{\;\;��\;\;}}{\;}$2Cu+2HCl��
�ܵ��CuCl2��Һʱ�������ϲ�����ɫ���ʵ�ԭ���õ缫��Ӧʽ����ΪCu2++e-+Cl-=CuCl����
�������ϲ�����ɫ���ʵ����ʵ�����$\frac{{w}_{1}-{w}_{2}}{35.5}$mol����װ��b��ȴʱ������ͨH2�����������CuCl�IJ��ʻ�ƫС��ƫ��ƫС�����䣩��
| A�� | 3��1 | B�� | 9��2 | C�� | 2��1 | D�� | 4��1 |
 �״���һ�����͵���Դ��
�״���һ�����͵���Դ��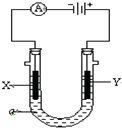 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺