��Ŀ����
����Ŀ����1��������һ��ǿ�ᣬ�����Ũ�ȳ���40%�ͻ�Ѹ�ٷֽ⣬��Ӧ�Ļ�ѧ����ʽΪ��8HClO3=3O2����2Cl2����4HClO4��2H2O�����������������С�⣺
���������仯�����У�������ԭ��Ӧ�Ĺ�����______________��______________���ѧʽ����
���÷�Ӧ������������____________���ѧʽ���������û�������ƽ����Է���������
��2����֪�ⶨ�̵�һ�ַ����ǣ�������ת��Ϊ����������ӣ���Ӧ��ϵ����H����Mn2����H2O��IO![]() ��MnO
��MnO![]() ��IO
��IO![]() ��
��
���йط�Ӧ�����ӷ���ʽΪ__________________��
����������ת��Ϊ����������ӵķ�Ӧ�У�����ѷ�Ӧ�����Һϡ�͵�1 L�������Һ��pH��2�����ڷ�Ӧ��ת�Ƶ��ӵ����ʵ��� mol��
���𰸡�
��1��HClO3��Cl2��O2��HClO4��47.6��
��2��2Mn2++5IO4-+3H2O��2MnO4-+5IO3-+6H+��1/60����0.0167����
��������
�����������1��8HClO3��3O2��+2Cl2��+4HClO4+2H2O�У��������ͻ�ԭ������HClO3��HClO3��ClԪ�صõ��ӻ��ϼ۽�������Cl2��������ԭ��Ӧ�����Է�����ԭ��Ӧ�Ĺ�����HClO3��Cl2��O��Ԫ�صĻ��ϼ���-2����0�ۣ�ClԪ�صĻ��ϼ���+5����+7�ۣ���������������O2��HClO4��ƽ��ʽ��=ƽ��Ħ������=![]() =
=![]() =47.6���ʴ�Ϊ��HClO3��Cl2��O2��HClO4��47.6��
=47.6���ʴ�Ϊ��HClO3��Cl2��O2��HClO4��47.6��
��2����������ʧ���ӱ��������ɸ���������ӣ���������������ԭ�����������õ��ӻ��ϼ۽��ͣ�IO3-��IO4-�е�Ԫ�صĻ��ϼ۷ֱ���+5�ۺ�+7�ۣ�����IO4-������������ԭ������IO3-��ͬʱˮ�μӷ�Ӧ���������ӣ����Ը÷�Ӧ�����ӷ���ʽΪ��2Mn2++5IO4-+3H2O��2MnO4-+5IO3-+6H+�������÷�Ӧ��Ƴ�ԭ��أ������ϵõ��ӻ��ϼ۽��Ͷ�������ԭ��Ӧ�������������ɵ������ǵ�������ӣ��ʴ�Ϊ��2Mn2++5IO4-+3H2O��2MnO4-+5IO3-+6H+��
����������ת��Ϊ����������ӵķ�Ӧ�У�����ѷ�Ӧ�����Һϡ�͵�1 L�������Һ��pH��2��c��H+��=10-2mol/L��n��H+��=10-2mol/L��1L=0.01mol������2Mn2++5IO4-+3H2O��2MnO4-+5IO3-+6H+��֪��ת�Ƶ��ӵ����ʵ���Ϊ0.01mol��![]() ��2��5=
��2��5=![]() mol���ʴ�Ϊ��
mol���ʴ�Ϊ��![]() ��
��

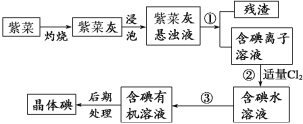
����Ŀ��
�ɷֵ�����/g | Ħ������/��gmol��1�� | |
���� | 25.00 | 342 |
����� | 0.87 | 174 |
��˾ƥ�� | 0.17 | 180 |
������� | 0.316 | 158 |
������ | 0.02 | 170 |
��1���������ʻ����ʼ�����K������˾ƥ���в���K+�������ʵ���Ũ��Ϊ__________molL������Ҫ����ԭʼ����д������ʽ���ɣ�����Ҫ��������㣩��
��2�������������ʻ����ʼ���������������ձ�����������ҩ�ס�������ƽ�������룩��
____________________������ȱ���������ƣ���
��3������Һ���ƹ����У����в��������ƽ��û��Ӱ�����_________������ĸ����
A������ʱ����������ƿ�̶���
B������ƿ��ʹ��ǰδ�����������������ˮ
C������ƿ��ʹ��ǰ�ո�������һ�����ʵ���Ũ�ȵ�NaCl��Һ��δϴ��
D������ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ���δ���κδ���
��4����Ҫ����0.5mol��L��1500ml��������Һ����
��������������Ϊ98%���ܶ�Ϊ1.84g��cm��3��Ũ��������Ϊ___________������������һλС����mL;
�����ʵ������15mL��20mL��50mL��Ͳ��Ӧѡ��___________mL��Ͳ���;
�����ƹ������������ձ��н�Ũ����ϡ�ͣ�ϡ��ʱ����������_____________________.