��Ŀ����
��һ���¶���,���ݻ�����������м���2 mol N2��8 mol H2 ���������,ʹ֮��Ӧ.��֪:N2(g)+3H2(g)
 2NH3(g)����H=-92.2
kJ��mol-1.ƽ��ʱ,����������ѹǿΪ��ʼʱ��80%.
2NH3(g)����H=-92.2
kJ��mol-1.ƽ��ʱ,����������ѹǿΪ��ʼʱ��80%.
(1)��Ӧ�ﵽƽ��ʱ,�ų�������_______.
A.��92.2 kJ B.����92.2 kJ C.����92.2 kJ
(2)��ʹH2��ת�������Ϊԭ��������,����������������������,Ӧ��N2�ij�ʼ����2mol�����__________mol.
(3)����ͬһ�¶�,����ͬ��������,����ʼʱ����2molNH3��1molH2 ���������,��Ӧ�ﵽƽ��ʱNH3���������_________.
A.����0.25 B.����0.25 C.��0.25
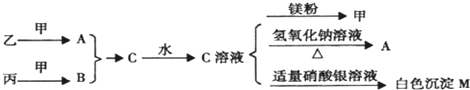
(4)��ͼ��T1��ʱ������NH3�����ʵ�����ʱ��ı仯����,���ڸ�ͼ�в������÷�Ӧ��T2��(T2>T1)ʱn(NH3)�ı仯����.
���𰸡�
��1��B ��2��64.5 ��3��C ��4����
����������

��ϰ��ϵ�д�
�����Ŀ
��һ���¶��£����ݻ�Ϊ2L���ܱ������г���һ������CO��NO2������Ӧ��4CO��g��+2NO2��g��?N2��g��+4CO2��g����H��0���йظ÷�Ӧ��������ȷ���ǣ�������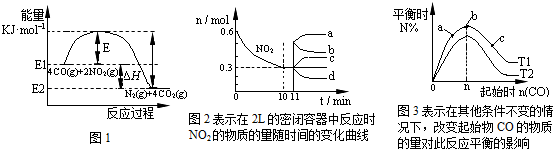

| A����һ���¶ȡ��̶��ݻ����ܱ���������������ܶȲ��ٸı����˵���������淴Ӧ�Ѵ�ƽ�� | B����ͼ1�ɵü����ʵ��Ĵ�����E�͡�H����С | C��ͼ2��0��10min�ڸ÷�Ӧ��ƽ������v��CO��=0.03mol?L-1?min-1����11min�������������䣬ѹ�����������Ϊ1L����n��NO2���ı仯����Ϊd | D��ͼ3��T1��T2��ʾ�¶ȣ���Ӧ�¶��µ�ƽ�ⳣ��ΪK1��K2����T1��T2��K1��K2 |