��Ŀ����
����Ŀ�������������ƣ�Na2S2O4���׳Ʊ��շۣ���һ��ǿ��ԭ������ҵ�Ͽ�ͨ�����մ�����Ⱦ�����SO2��NO����ȡ����ͬʱ���ɵõ�NH4NO3��Ʒ����������ͼ���£�CeΪ��Ԫ�أ���

��ش��������⣺
(1)װ��I�п�����NaHSO3��������NaHSO3��Һ�����Ե�ԭ����____________��
(2)װ��II�����������£�NO��Ce4+����ΪNO3- ʱ�����������뻹ԭ��������ʵ���֮��Ϊ______��
(3)װ��III�������ϵĵ缫��ӦʽΪ__________����װ��IV��NO2- ��Ũ��Ϊ11.5g��L-1Ҫʹ1dm3��
��Һ�е�NO2- ��ȫת��ΪNH4NO3��������װ��IV��ͨ���״���µ�O2__________L��
(4)Na2S2O4�ڿ��������ױ��������䷴Ӧ����ʽ����Ϊ��
��2Na2S2O4+O2+2H2O=4NaHSO3��
��Na2S2O4+O2+H2O=NaHSO3+ NaHSO4��
�����ʵ��֤������ʱһ���з�Ӧ�ڷ���______________��
(5)SO2���������ӽ���Ĥȼ�ϵ��ԭ��ʾ��ͼ����ͼ��

���ӵ���������Ϊ__________������A��B����B��A�����������ĵ缫��ӦʽΪ_____________��
���𰸡�HSO3-����Һ�д��ڵ���ƽ���ˮ��ƽ�⣺HSO3-![]() SO32-+H+��H2O
SO32-+H+��H2O![]() H2SO3+OH-������HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�����Һ�е�C(H+) >c(OH-)����Һ������1: 32HSO3- +2e- +2H+ =S2O42-+2H2O2.8ȡ������������ˮ�У�����BaCl2��Һ���а�ɫ������������֤��������A��BSO2-2e- +2H2O = SO4-+4H+
H2SO3+OH-������HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�����Һ�е�C(H+) >c(OH-)����Һ������1: 32HSO3- +2e- +2H+ =S2O42-+2H2O2.8ȡ������������ˮ�У�����BaCl2��Һ���а�ɫ������������֤��������A��BSO2-2e- +2H2O = SO4-+4H+
��������(1)��NaHSO3��Һ��HSO3-��������ˮ�⣺HSO3-![]() SO32-+H+��H2O
SO32-+H+��H2O![]() H2SO3+OH-������HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�����Һ�е�C(H+) >c(OH-)����Һ������
H2SO3+OH-������HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�����Һ�е�C(H+) >c(OH-)����Һ������
(2)NO������Ϊ�������Ce4+����ԭΪCe3+�����Ի�����ȱ����ˮ��ȱ�ⲹ�����ӣ���Ӧ�����ӷ���ʽΪNO+2H2O+3Ce4+=3Ce3++NO3-+4H+����������NO3-�뻹ԭ����Ce3+�����ʵ���֮��Ϊ1:3��
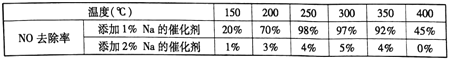
(3)��������������ԭ��Ӧ��SO32-����ԭΪS2O42-���缫��ӦʽΪ��2SO32-+2H2O-2e-�TS2O42-+4OH-��NO2-��Ũ��Ϊ11.5gL-1��Ҫʹ1dm3����Һ�е�NO2-��ȫת��ΪNH4NO3����ʧȥ������Ŀ�ǣ�![]() ��2�������ı���������������V����õ�������Ŀ�ǣ�
��2�������ı���������������V����õ�������Ŀ�ǣ�![]() ��4�����ݵ����غ㣺
��4�����ݵ����غ㣺![]() ��2=
��2=![]() ��4�����V=2.8L��
��4�����V=2.8L��
(4)ȡ������������ˮ�У�����BaCl2��Һ���а�ɫ�����������˳���ΪBaSO4��˵����Һ�к���SO42-������֤�� ����ʱһ���з�Ӧ��������
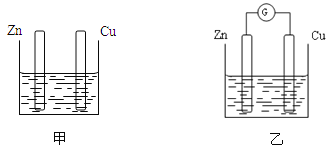
(5)����������������Ӧ������������ԭ��Ӧ�����Զ����������ڵ缫Ϊ�������������ڵ缫Ϊ������ԭ������������������������������ƶ�����Ϊ����A��B��������SO2������ΪSO42-ʱ�����ĵ缫��ӦΪSO2-2e- +2H2O = SO42-+4H+��

����Ŀ����̼����(2Na2CO3��3H2O2)��һ�ּ�ϴ�ӡ�Ư�ס�ɱ����һ�����ϵƯ����ij��ȤС���Ʊ���̼���Ƶ�ʵ�鷽����װ��ʾ��ͼ���£�

��֪������Ӧ��2Na2CO3(aq) + 3H2O2(aq)![]() 2Na2CO3��3H2O2(s) ��H < 0
2Na2CO3��3H2O2(s) ��H < 0
����Ӧ��2H2O2= 2H2O + O2����50��ʱ2Na2CO3��3H2O2(s) ��ʼ�ֽ⡣

��ش��������⣺
��1���������Ĺؼ��� ��ԭ���� ��
��2������ҺX�м�������NaCl����Ŀ����_________________��
��3����������ѡ����ˮ�Ҵ�ϴ�Ӳ�Ʒ��Ŀ���� ��
��4�����������У��������̼����ʧЧ���� ��
A��NaHCO3 | B��Na2SO3 | C��Na2SiO3 | D��HCl |
��5����̼���Ʋ�Ʒ��������������̼���ƣ������������ⶨ��̼���Ƶ�������������������裺ȡ��Ʒ�ܽ�������BaCl2��Һ��������ϴ������������������Ҫֱ�Ӳⶨ���������У� (����ĸ��ʾ��ע���京��)����Ʒ�й�̼�������������ı���ʽΪ�� ��