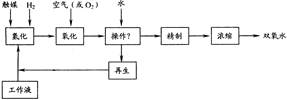
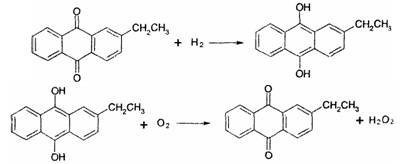
��Ŀ����
ij��ѧ����С���ͬѧ����ԭ���ԭ��̽��һ���¶��£�ʵ��ʱʵ�ʵĻ����¶ȣ�ʹ���ۻ�����������Ũ�ȡ�
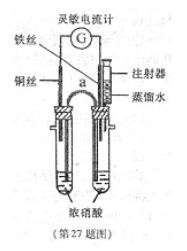
ʵ�鲽�����£�
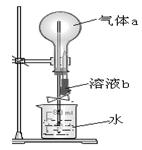
������֧��֧�Թ��зֱ����Ũ����5.0mL����������Ϊ65%��
�ܶ�Ϊ1.4g/mL������ͼ��װ��ʵ��װ�á�
�ڽ�ͭ˿����˿ͬʱ����Ũ�����У��۲쵽����������ָ����ָ��ͭ˿������Ѹ�ٷ�ת��ֻ��1��2s��ָ����˿һ�ˡ�
����ʢ��5.0mL����ˮ��ע�������֧�Թ��ڼ�ˮ����ע�ӵ����Ƶ�ָ��ƫת������ָ��ǡ�÷�����ת��ָ��ͭ˿ʱֹͣ
ʵ�飬��¼��ʱ��ע���ˮ�������
�ظ�����ʵ���õ��������£�
��ش��������⣺
��1���ڸ�װ��������a�������ö���Ϊ ��ָ��ָ����˿ʱ����˿Ϊ ���������������
��2��65%����������ʵ���Ũ���� ����δעˮǰ�����ĵ缫��ӦʽΪ ��
��3��T1 T2���>������<����=����
��4���ڱ�ʵ���¶��£�ʹ���ۻ����������Ũ��Ϊ %�����ñ�ʵ�������жϣ�����45%���������ʵ�飨����עˮ�������������ָ��Ӧָ�� �缫���������ͭ����
��5����ʵ��װ���ϵIJ����� ���Ľ������� ��

ʵ�鲽�����£�
������֧��֧�Թ��зֱ����Ũ����5.0mL����������Ϊ65%��
�ܶ�Ϊ1.4g/mL������ͼ��װ��ʵ��װ�á�
�ڽ�ͭ˿����˿ͬʱ����Ũ�����У��۲쵽����������ָ����ָ��ͭ˿������Ѹ�ٷ�ת��ֻ��1��2s��ָ����˿һ�ˡ�
����ʢ��5.0mL����ˮ��ע�������֧�Թ��ڼ�ˮ����ע�ӵ����Ƶ�ָ��ƫת������ָ��ǡ�÷�����ת��ָ��ͭ˿ʱֹͣ
ʵ�飬��¼��ʱ��ע���ˮ�������
�ظ�����ʵ���õ��������£�
| ʵ����� | ʵ���¶ȣ��棩 | ע��ˮ�������mL�� |
| 1 | 17.2 | 2.4 |
| 2 | T1 | 2.5 |
| 3 | T2 | 2.3 |
��1���ڸ�װ��������a�������ö���Ϊ ��ָ��ָ����˿ʱ����˿Ϊ ���������������
��2��65%����������ʵ���Ũ���� ����δעˮǰ�����ĵ缫��ӦʽΪ ��
��3��T1 T2���>������<����=����
��4���ڱ�ʵ���¶��£�ʹ���ۻ����������Ũ��Ϊ %�����ñ�ʵ�������жϣ�����45%���������ʵ�飨����עˮ�������������ָ��Ӧָ�� �缫���������ͭ����
��5����ʵ��װ���ϵIJ����� ���Ľ������� ��

��

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
�����Ŀ
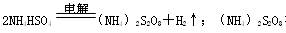 ��Һ����ˮ�����м�ѹˮ�⡢�������������������ˮ��Һ��ʣ����Һ������������ѭ��ʹ�á�
��Һ����ˮ�����м�ѹˮ�⡢�������������������ˮ��Һ��ʣ����Һ������������ѭ��ʹ�á�