��Ŀ����
������ƿδ֪��Һ�����ǣ�NaCl(0.1mol/L)��NaOH��0.1mol/L����HCl(0.1mol/L)����̪(0.1mol/L)��ij��ѧ����С�飬Ϊ�˽����Ǽ������������û�������κ��Լ�������ͬѧ�Dz�ȡ���µ�ʵ�鲽�裺
��1������ƿ��Һ��A��B��C��D���
��2��ȡ������Һ������ϣ�����ʵ�������������ʷֳ�����
��3��ȡ������Һ�����ϵ��������е�һ��δ֪Һ�����Լ���� A�� B��
��4����ȡ�Ѽ��������Һ2ml������3��C��Һ���ٵμ�D��Һ4ml�������������������Ե�ʵ������֪C D ��д��ѧʽ��д������ʵ������з�Ӧ�����ӷ���ʽ
��1������ƿ��Һ��A��B��C��D���
��2��ȡ������Һ������ϣ�����ʵ�������������ʷֳ�����
��3��ȡ������Һ�����ϵ��������е�һ��δ֪Һ�����Լ���� A�� B��
��4����ȡ�Ѽ��������Һ2ml������3��C��Һ���ٵμ�D��Һ4ml�������������������Ե�ʵ������֪C D ��д��ѧʽ��д������ʵ������з�Ӧ�����ӷ���ʽ
A ��HCl��B��NaCl��C����̪��D ��NaOH H++OH-��H2O
������Һ����������Ͽɲ������в�ͬ������NaOH��Һ��̪��Ϻ���Һ�ʺ�ɫ��NaCl��HCl�����Һ��ɫ����NaOH�ͷ�̪�Ļ��Һ�еμ�HCl��Һ����ɫ��ʧ��������NaOHʱ�������Ӷ��ɵij�HCl��NaCl������ȡ2ml0.1mol/l�����ᣬ���ȵ�3��0.1mol/lNaOH��Һ���ٵμ�4ml��̪����Һ���ɫ����˼����NaOH��Һ�ͷ�̪��

��ϰ��ϵ�д�
 �ŵ������ϵ�д�
�ŵ������ϵ�д�
�����Ŀ
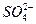 ��
��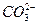 ��OH-��Cl-����ֻ����ȡ���θ���Һ�����������ӷֱ��������������IJ���˳��ӦΪ��
��OH-��Cl-����ֻ����ȡ���θ���Һ�����������ӷֱ��������������IJ���˳��ӦΪ��