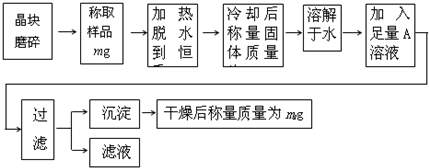
��Ŀ����
Ϊ�˲ⶨ��������KCl���ʵ�����KAl��SO4��2?nH2O�Ĵ��ȣ�ͬʱ�ⶨn��ֵ�����������̽���ʵ�飺

��1������ĥ��ʱʹ�õ���������
��2�������ȹ������й���ɽ����������ʧ�����ʹ��õ�nֵƫ
��3���ڼ��Ƚ����������ȴ���壿
��4��A��Һ��
��5�����˺��轫����ϴ�Ӹɾ���ϴ�ӷ�����
��6��ͨ��ʵ�飬���������ѧʽ��nֵΪ12���������Ĵ���Ϊ
%��

��1������ĥ��ʱʹ�õ���������
�в�
�в�
����2�������ȹ������й���ɽ����������ʧ�����ʹ��õ�nֵƫ
��
��
���ж�����ˮ�����صķ��������γ������������0.1g
��������������0.1g
����3���ڼ��Ƚ����������ȴ���壿
������ǯ�������Ƶ�����������ȴ
������ǯ�������Ƶ�����������ȴ
����4��A��Һ��
BaCl2
BaCl2
���ѧʽ�����ж�A��Һ�Ƿ������ķ��������ú����ϲ���Һ�μ�����A�۲�����������
���ú����ϲ���Һ�μ�����A�۲�����������
����5�����˺��轫����ϴ�Ӹɾ���ϴ�ӷ�����
�ò�����������������ˮ����û���������ˮ������ظ����ϲ�����ϴ��Ϊֹ
�ò�����������������ˮ����û���������ˮ������ظ����ϲ�����ϴ��Ϊֹ
����6��ͨ��ʵ�飬���������ѧʽ��nֵΪ12���������Ĵ���Ϊ
| 23700m2 |
| 233m |
| 23700m2 |
| 233m |
��������1��ʹ���в����������飻
��2������ʱ����ɽ�������ʣ����������ƫС���ⶨˮ������ƫ�ߣ�
������ƽ��ȷ��0.1���ʵ����γ��������������0.1gʱ������Ϊ���������أ���ȫ��ˮ��
��3��Ϊ��ֹ�������տ����е�ˮ���������ɽᾧˮ���Ӧ�ڸ��������ȴ��
��4��������ͼ��֪��������Ʒ�Ĵ��ȣ��ɼ����Ȼ�����Һ�������������ᱵ�������ⶨ��Ʒ�Ĵ��ȣ�
�ж���Һ�Ƿ������ķ����Ǿ��ø��ϲ���Һ�μ�����A�۲�������������˵���Ѿ�������
��5��ϴ�ӳ����ķ����ǣ��ò�����������������ˮ����û���������ˮ������ظ����ϲ�����ϴ��Ϊֹ��
��6�������������ᱵ�����������������������Ӷ�������Ʒ�Ĵ��ȣ�
��2������ʱ����ɽ�������ʣ����������ƫС���ⶨˮ������ƫ�ߣ�
������ƽ��ȷ��0.1���ʵ����γ��������������0.1gʱ������Ϊ���������أ���ȫ��ˮ��
��3��Ϊ��ֹ�������տ����е�ˮ���������ɽᾧˮ���Ӧ�ڸ��������ȴ��
��4��������ͼ��֪��������Ʒ�Ĵ��ȣ��ɼ����Ȼ�����Һ�������������ᱵ�������ⶨ��Ʒ�Ĵ��ȣ�
�ж���Һ�Ƿ������ķ����Ǿ��ø��ϲ���Һ�μ�����A�۲�������������˵���Ѿ�������
��5��ϴ�ӳ����ķ����ǣ��ò�����������������ˮ����û���������ˮ������ظ����ϲ�����ϴ��Ϊֹ��
��6�������������ᱵ�����������������������Ӷ�������Ʒ�Ĵ��ȣ�
����⣺��1��ʹ���в����������飻
�ʴ�Ϊ���в���
��2������ʱ����ɽ�������ʣ����������ƫС���ⶨˮ������ƫ�ߣ��ʲⶨnֵƫ�ߣ�
������ƽ��ȷ��0.1���ʵ����γ��������������0.1gʱ������Ϊ���������أ���ȫ��ˮ��
�ʴ�Ϊ���ߣ����γ������������0.1 g��
��3��Ϊ��ֹ�������տ����е�ˮ���������ɽᾧˮ���Ӧ�ڸ��������ȴ��ע��������ǯ�г֣�
�ʴ�Ϊ��������ǯ�������Ƶ�����������ȴ��
��4��������Ʒ�Ĵ��ȣ��ɼ����Ȼ�����Һ�������������ᱵ�������ⶨ��Ʒ�Ĵ��ȣ��ж���Һ�Ƿ������ķ����Ǿ��ú����ϲ���Һ�μ�����A�۲�������������˵���Ѿ�������
�ʴ�Ϊ��BaCl2�������Լ�ֻҪ����Ҳ���ԣ������ú����ϲ���Һ�μ�����A�۲�������������
��5��ϴ�ӳ����ķ����ǣ��ò�����������������ˮ����û���������ˮ������ظ����ϲ�����ϴ��Ϊֹ��
�ʴ�Ϊ���ò�����������������ˮ����û���������ˮ������ظ����ϲ�����ϴ��Ϊֹ��
��6��������������ᱵ������Ϊm2g�������ᱵ�����ʵ���Ϊ
=
mol����KAl��SO4��2?12H2O������Ϊ
mol��
��474g/moL=
g�����������Ĵ���Ϊ
��100%=
%��
�ʴ�Ϊ��
��
�ʴ�Ϊ���в���
��2������ʱ����ɽ�������ʣ����������ƫС���ⶨˮ������ƫ�ߣ��ʲⶨnֵƫ�ߣ�
������ƽ��ȷ��0.1���ʵ����γ��������������0.1gʱ������Ϊ���������أ���ȫ��ˮ��
�ʴ�Ϊ���ߣ����γ������������0.1 g��
��3��Ϊ��ֹ�������տ����е�ˮ���������ɽᾧˮ���Ӧ�ڸ��������ȴ��ע��������ǯ�г֣�
�ʴ�Ϊ��������ǯ�������Ƶ�����������ȴ��
��4��������Ʒ�Ĵ��ȣ��ɼ����Ȼ�����Һ�������������ᱵ�������ⶨ��Ʒ�Ĵ��ȣ��ж���Һ�Ƿ������ķ����Ǿ��ú����ϲ���Һ�μ�����A�۲�������������˵���Ѿ�������
�ʴ�Ϊ��BaCl2�������Լ�ֻҪ����Ҳ���ԣ������ú����ϲ���Һ�μ�����A�۲�������������
��5��ϴ�ӳ����ķ����ǣ��ò�����������������ˮ����û���������ˮ������ظ����ϲ�����ϴ��Ϊֹ��
�ʴ�Ϊ���ò�����������������ˮ����û���������ˮ������ظ����ϲ�����ϴ��Ϊֹ��
��6��������������ᱵ������Ϊm2g�������ᱵ�����ʵ���Ϊ
| m2g |
| 233g/mol |
| m2 |
| 233 |
| m2 |
| 233 |
| 1 |
| 2 |
| 237m2 |
| 233 |
| ||
| mg |
| 23700m2 |
| 233m |
�ʴ�Ϊ��
| 23700m2 |
| 233m |
���������⿼�����ʵ���ɺͺ����IJⶨ����Ŀ���ѣ�����ע��ʵ���ԭ���Ͳ���������ע�������Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������

��ϰ��ϵ�д�
 �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д� �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
�����Ŀ