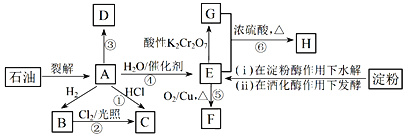
��Ŀ����
����Ŀ����֪:��NH3����ԭNOxʱ�������·�Ӧ.
��Ӧ��:4NH3 (g)+6NO(g) ![]() 5N2(g)+6H2O(l) H1=-1 807. 0 kJ��mol��1,
5N2(g)+6H2O(l) H1=-1 807. 0 kJ��mol��1,
��Ӧ��:4NH3(g)+6NO2(g) ![]() 5N2(g)+3O2(g)+6H2O(l) H2=?
5N2(g)+3O2(g)+6H2O(l) H2=?
��Ӧ��:2NO(g)+O2(g) ![]() 2NO2(g) H3=-113.0kJ��molһ1
2NO2(g) H3=-113.0kJ��molһ1
(1)��Ӧ�ڵ�H2==_____________��
(2)Ϊ̽���¶ȼ���ͬ�����Է�Ӧ�ٵ�Ӱ��.�ֱ��ڲ�ͬ�¶ȡ���ͬ������.����������ʼ���������ظ�ʵ��.����ͬʱ���ڲ��N2Ũ�ȵı仯�������ͼ��ʾ��
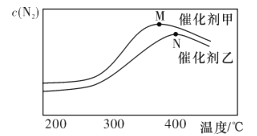
�ٷ�Ӧ�ٵ�ƽ�ⳣ���ı���ʽK=________����ͬ�¶���.�ڴ����������·�Ӧ��ƽ�ⳣ��______(����ڡ���С�ڡ����ڡ�)�ڴ����ҵ������·�Ӧ��ƽ�ⳣ����
��N���N2Ũ�ȼ�С��ԭ�������_____________________��
(3)ij�¶��£���1 L�����ܱ������г�ʼͶ��4 mol NH3��6 mol NO������Ӧ��.�����������ʵ���Ϊ7.5molʱ��Ӧ�ﵽƽ��.��NH3��ת����Ϊ____����ƽ������ʱ��Ϊ5 min.����NO��ʾ�˷�Ӧ0��5 min�ڵ�ƽ����Ӧ����Ϊ______.
���𰸡�-1468.0kJ/mol 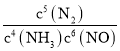 ���� �¶����߷�������Ӧ���¶����ߴ������Խ��� 50% 0.6mol/(L��min)
���� �¶����߷�������Ӧ���¶����ߴ������Խ��� 50% 0.6mol/(L��min)
��������
��1���ɸ�˹������Ӧ�ڵ�H2 ��
��2���ٸ���ƽ�ⳣ���Ķ��壬д����Ӧ��4NH3(g) + 6NO(g) ![]() 5N2(g) + 6H2O(l) ��ƽ�ⳣ���ı���ʽK=c5(N2)/ c4(NH3) c6(NO)��������Ӱ��ƽ�ⳣ����
5N2(g) + 6H2O(l) ��ƽ�ⳣ���ı���ʽK=c5(N2)/ c4(NH3) c6(NO)��������Ӱ��ƽ�ⳣ����
��N���N2Ũ�ȼ�С��ԭ��������¶����߷�������Ӧ�� �¶����ߴ������Խ��ͣ�
��3���г�����ʽ���м��㡣
��1����Ӧ�٣�4NH3(g) + 6NO(g) ![]() 5N2(g) + 6H2O(l) H1 = -1807.0kJmol-1
5N2(g) + 6H2O(l) H1 = -1807.0kJmol-1
��Ӧ�ڣ�4NH3(g) + 6NO2(g) ![]() 5N2(g) + 3O2(g) + 6H2O(l) H2 = ?
5N2(g) + 3O2(g) + 6H2O(l) H2 = ?
��Ӧ�ۣ�2NO(g) + O2(g) ![]() 2NO2(g) H3 = -113.0kJmol-1
2NO2(g) H3 = -113.0kJmol-1
�ɸ�˹���ɣ���Ӧ��-��Ӧ����3���÷�Ӧ�ڵ�H2=-1807.0kJmol-1-��-113.0kJmol-1����3=-1468.0kJ/mol��
��2���ٸ���ƽ�ⳣ���Ķ��壬д����Ӧ�ٵ�ƽ�ⳣ���ı���ʽK= ��������Ӱ��ƽ�ⳣ�����¶Ȳ��䣬ƽ�ⳣ�����䣬�ʼ������ִ��������£���Ӧ��ƽ�ⳣ����ȣ�
��������Ӱ��ƽ�ⳣ�����¶Ȳ��䣬ƽ�ⳣ�����䣬�ʼ������ִ��������£���Ӧ��ƽ�ⳣ����ȣ�
��N���N2Ũ�ȼ�С��ԭ��������¶����߷�������Ӧ�� �¶����ߴ������Խ��ͣ�
��3���г�����ʽ��
4NH3(g) + 6NO(g) ![]() 5N2(g) + 6H2O(l)
5N2(g) + 6H2O(l)
cʼ/mol��L��1 4 6
cת/mol��L��1 4x 6x 5x
cƽ/mol��L��1 4-4x 6-6x 5x
4-4x +6-6x+5x=7.5mol/1L��x=0.5����NH3��ת����50% ��
��ƽ������ʱ��Ϊ5���ӣ�����NO��ʾ�˷�Ӧƽ����Ӧ����Ϊv(NO)=![]() =0.6mol/(L�� min)��
=0.6mol/(L�� min)��

 �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�����Ŀ�����и�����I��II������Ӧ����ͬһ��Ӧ���͵��ǣ� ��
ѡ�� | ��ӦI | ��ӦII |
A |
| CH2=CH2��CH3CH2Cl |
B | CH3CH2Cl��CH3CH2OH | CH3CH2OH��CH3COOCH2CH3 |
C | CH3CH2OH��CH2=CH2 | CH3CH2OH��CH3CHO |
D | ��֬������ |
|
A. AB. BC. CD. D