��Ŀ����
����Ŀ��H2C2O4 (��������Ԫ����)���ڱ���������ؾ�����Ϊ�궨NaOH��ҺŨ�ȵĻ����ʣ��Ӷ����NaOH����Һ��
��1����ˮ��Һ��H2C2O4�ĵ��뷽��ʽΪ________��
��2����0.1molL-1NaOH��Һ�ζ�0.1molL-1������Һ�ĵζ���������ͼ��ʾ��

�ٵζ������д�X�㵽Y�㣬��Ӧ�����ӷ���ʽΪ________��
����NaOH��Һ�ζ�������������������ƿ����Һ��ˮ�ĵ���̶� _______(�����)��
a.ʼ�ռ�С b.ʼ������ c.�ȼ�С������ d.��������С
��X��ʱ��c(Na+)-c(C2O42-)_____c(H2C2O4) +c(HC2O4-) (ѡ������������=);Y��ʱ��c(OH-) - c(H+) _______c(H2C2O4)+ c(HC2O4-) (ѡ������������=)��
��3��ijͬѧ���ڱ����������(![]() ��Ħ������Ϊ204gmol-1��������ˮ�Ĺ��壬ˮ��Һ��������)�궨NaOH��Һ����Ҫʵ�鲽��������
��Ħ������Ϊ204gmol-1��������ˮ�Ĺ��壬ˮ��Һ��������)�궨NaOH��Һ����Ҫʵ�鲽��������
������.ȷ��ȡ0.4896g�ڱ��������������ƿ�У���������ˮ�ܽ�
��.����1~2�η�̪��ָʾ��
��.�ô���NaOH��Һ�ζ�����
���жϴﵽ�ζ��յ�ķ�����___________��
�����ζ����յ�����NaOH��ҺΪ25.00mL����ôεζ���õ�NaOH��ҺŨ��Ϊ _______��
����������ᵼ�²�õ�NaOH��ҺŨ��ƫ�����__________(�����)��
a.�ζ���δ�ô���NaOH��Һ��ϴ
b.�ζ�ǰ�ζ��ܼ�˲��������ݣ��ζ���������ʧ
c.�ζ���������������ˮϡ����ƿ����Һ
d.����ʱ���ζ�ǰ���ӵζ��̶ܿȣ��ζ���ƽ�ӵζ��̶ܿ�
���𰸡� H2C2O4![]() H++HC2O4-��HC2O4-
H++HC2O4-��HC2O4- ![]() H++C2O42- HC2O4-+OH-=C2O42-+H2O d = �� �μ����һ��NaOH��Һʱ����ƿ����Һ����ɫ���Ұ�����ڲ���ɫ 0.09600molL-1 d
H++C2O42- HC2O4-+OH-=C2O42-+H2O d = �� �μ����һ��NaOH��Һʱ����ƿ����Һ����ɫ���Ұ�����ڲ���ɫ 0.09600molL-1 d
��������(1)�����Ƕ�Ԫ���ᣬ��ˮ��Һ������������룬��һ������̶ȴ��ڵڶ���������뷽��ʽ�ֱ�Ϊ��H2C2O4![]() HC2O4-+H+��HC2O4-
HC2O4-+H+��HC2O4-![]() C2O42-+H+���ʴ�Ϊ��H2C2O4
C2O42-+H+���ʴ�Ϊ��H2C2O4![]() HC2O4-+H+��HC2O4-
HC2O4-+H+��HC2O4-![]() C2O42-+H+��
C2O42-+H+��
(2)�ٵζ������д�X�㵽Y�㣬Ϊ�������ƺ��������Ʒ�Ӧ�����ӷ�ӦΪ��HC2O4-+OH-=C2O42-+H2O���ʴ�Ϊ��HC2O4-+OH-=C2O42-+H2O��
����NaOH��Һ�ζ�����ʼ��Һ�����ԣ��ζ�����������������Ũ�ȼ�С��ˮ�ĵ���̶������ζ���ȫ��NaOH��Һ�ζ�����������Һ�ʼ��ԣ�����ˮ�ĵ��룬��������������ƿ����Һ��ˮ�ĵ���̶�ʹ��������С���ʴ�Ϊ��d��
��X��ʱ��ҺΪNaHC2O4��Һ��HC2O4-����Һ�з���������ˮ�⣬����ƽ�⣺HC2O4-+H2OH2C2O4+OH-��̼Ԫ������Һ�д�����ʽ�У�HC2O4-��H2C2O4��C2O42-�����������غ���c(Na+)=c(HC2O4-)+c(H2C2O4)+c(C2O42-)����c(Na+)-c(C2O42-)=c(H2C2O4)+c(HC2O4-)��Y��ʱΪNa2C2O4��Һ�����ݵ���غ��У�c(Na+)+c(H+)=c(HC2O4-)+2c(C2O42-)+c(OH-)�٣������غ���c(Na+)=2[c(HC2O4-)+c(H2C2O4)+c(C2O42-)]�ڣ����ڴ���ٵ�c(OH-)-c(H+)=c(H2C2O4)+2c(HC2O4-)������c(OH-)-c(H+)��c(H2C2O4)+c(HC2O4-)���ʴ�Ϊ��=������
(3)���ڱ����������Ϊ���ᣬ��̪��pH��8ʱΪ��ɫ��pHΪ8��10֮�䣬��dz��ɫ�������ô���NaOH��Һ�ζ����յ㣬����ɫ��Һ�����ɫ���Ұ�����ڲ���ɫ��˵����Ӧ���յ㣬�ʴ�Ϊ���μ����һ��NaOH��Һʱ����ƿ����Һ����ɫ���Ұ�����ڲ���ɫ��
��0.4896g![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() =0.0024mol��
=0.0024mol��
![]() +NaOH��
+NaOH��![]() +H2O
+H2O
1 1
0.0024mol 0.025L��c(NaOH)
��0.0024mol =0.025L��c(NaOH)����ã�c(NaOH)=0.09600 mol/L�ʴ�Ϊ��0.09600molL-1��
��a����ʽ�ζ�����ȡNaOH��Һʱ��δ������ϴ����������NaOH��ҺŨ�Ƚ��ͣ���a����b���ζ�ǰ�ζ��ܼ�˲��������ݣ��ζ��յ�ʱ������ʧ�����V(�����)ƫ����c(�����)= 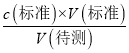 ������֪c(�����)ƫС����b����c���ζ���������������ˮϡ����ƿ����Һ����Һ�����ʵ������䣬����c(�����)=
������֪c(�����)ƫС����b����c���ζ���������������ˮϡ����ƿ����Һ����Һ�����ʵ������䣬����c(�����)= 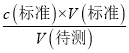 ������֪��c(����)���䣬��c����d������ʱ���ζ�ǰ���ӵζ��̶ܿȣ��ζ���ƽ�ӵζ��̶ܿȣ����V(�����)��Һ���ƫС������c(�����)=
������֪��c(����)���䣬��c����d������ʱ���ζ�ǰ���ӵζ��̶ܿȣ��ζ���ƽ�ӵζ��̶ܿȣ����V(�����)��Һ���ƫС������c(�����)= 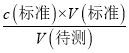 ������֪c(�����)ƫ��d��ȷ���ʴ�Ϊ��d��
������֪c(�����)ƫ��d��ȷ���ʴ�Ϊ��d��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�