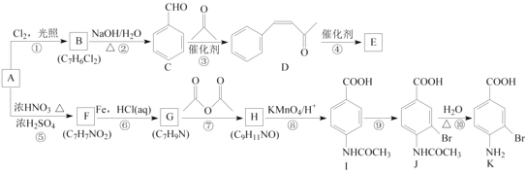
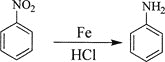
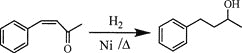
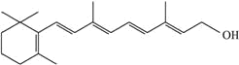
题目内容
【题目】(1)已知25℃、101 kPa时:
①2SO2(g)+O2(g)2SO3(g) ΔH1=-197 kJ/mol;
②H2O(g)=H2O(l) ΔH2=-44 kJ/mol;
③2SO2(g)+O2(g)+2H2O(g)=2H2SO4(l) ΔH3=-545 kJ/mol。
则SO3(g)与H2O(l)反应的热化学方程式为__________。
(2)已知:温度过高时,WO2(s)转变为WO2(g):
①WO2(s)+2H2(g)W(s)+2H2O(g) ΔH1=+66.0 kJ·mol-1
②WO2(g)+2H2(g)W(s)+2H2O(g) ΔH2=-137.9 kJ·mol-1
则WO2(s)WO2(g)的ΔH=__________。
【答案】SO3 (g)+H2O(l)=H2SO4(l) △H=-130kJ/mol +203.9 kJmol-1
【解析】
(1)2SO2(g)+O2(g)=2SO3(g)△H1=-197kJ/mol ①
H2O (g)=H2O(1)△H2=-44kJ/mol ②
2SO2(g)+O2(g)+2H2O(g)=2H2SO4(l)△H3=-545kJ/mol③
利用盖斯定律:(③-①)×![]() -②得SO3 (g)+H2O(l)=H2SO4(l)△H=-130kJ/mol;
-②得SO3 (g)+H2O(l)=H2SO4(l)△H=-130kJ/mol;
(2)已知:①WO2 (s)+2H2 (g)W (s)+2H2O (g);△H=+66.0kJmol-1
②WO2 (g)+2H2W (s)+2H2O (g);△H=-137.9kJmol-1
①-②得则WO2 (s)WO2 (g),故△H=66.0kJmol-1-(-137.9kJmol-1)=+203.9 kJmol-1。

【题目】醇与氢卤酸的反应是制备卤代烃的重要方法,实验室制备溴乙烷和1—溴丁烷的反应如下:NaBr+H2SO4(浓)=HBr+NaHSO4R-OH+HBr→R-Br+H2O
可能存在的副反应有:醇在浓硫酸的作用下脱水生成烯烃和醚,Br-被浓硫酸氧化为Br2等。有关数据列表如下:
乙醇 | 溴乙烷 | 正丁醇 | 1—溴丁烷 | |
密度/g.cm-3 | 0.79 | 1.46 | 0.81 | 1.28 |
沸点/℃ | 78.5 | 38.4 | 117.2 | 101.6 |
请回答下列问题:
(1)溴乙烷和1—溴丁烷的制备过程中,下列仪器最不可能用到的是(______)
A.圆底烧瓶 B.量筒 C.锥形瓶 D.漏斗
(2)卤代烃的水溶性__(填“大于”、“小于”或者“等于”)相应的醇,其原因是__。
(3)将溴乙烷粗产品置于分液漏斗中加水,震荡后静置,产品在__(填“上层”、“下层”或者“不分层”)。
(4)在制备溴乙烷时,乙醇会在浓硫酸的作用下脱水生成醚,写出此反应的化学方程式__。
(5)制备操作中,加入的浓硫酸必须稀释,其原因是(______)
A.减少副产物烯烃和醚的生成 B.减少Br2的生成
C.减少HBr的挥发 D.水是反应的催化剂
(6)欲除去卤代烃中的少量杂质Br2,下列物质中最适合的是(______)
A.NaI B.NaOH C.NaHSO3 D.KCl