��Ŀ����
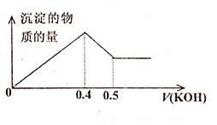
������ΪW1g���ơ��������Ͷ��һ������ˮ�г�ַ�Ӧ������û��ʣ�࣬���ռ�����״���µ�����V1L������Һ����μ���Ũ��Ϊa mol��L-1��HC1��Һ���������а�ɫ���������������ܽ⣬������ǡ����ʧʱ����HC1��Һ���ΪV2L������Һ���ɳ�����յõ�����W2g�����й�ϵʽ�д������ ��������
| A��24n��A1��+35��5n��Na��=W2��W1 | B��n��Na��+3n��A1��=aV2 |
| C��n��Na��+3n��A1��=V1/11��2 | D��aV2=V1/22��4 |
D
�𰸣�D
������һ���ۺ����ƺ����йػ�ѧ���ʵĽ��ѵļ�����ѡ���⣬��Ŀ�ں��˻�ѧ�е�"��̬--��̬"˼���Լ��ڻ�ѧѡ��ͼ��������ձ�ʹ�õ��غ㷨���������⣬Na������NaCl��ʽ���ڣ���������Ϊ35.5n(Na)��AlCl3�����ɹ�����ˮ��ΪAl(OH)3��HCl��HCl�ӷ���������ɲ���ΪAl2O3�����ӵ�����Ϊ24n��A1����24n��A1��+35��5n��Na��=W2��W1��A��ȷ��
W1 g��Na��Al������һ������ˮ��Ӧ���û�����V1 L H2���ɵ��ӵ�ʧ�غ�֪��Na��Al��ȫ��Ӧ���Na����Al3��ʧȥ�ĵ�����Ӧ�õ������ɵ�H2�õ��ĵ�����������n(Na)��3n(Al)��2V1/22.4��C��ȷ ;
;��Һ����һϵ�еķ�Ӧ���յõ�����ҺӦ����NaCl��AlCl3�Ļ�ϣ���ˣ�aV2�ɿ�����������ClԪ�ص����ʵ�������ClԪ���غ�֪aV2=n(Na)��3n(Al)��2V1/22.4��B��ȷ��D����

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ

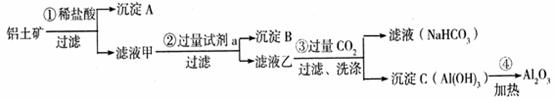
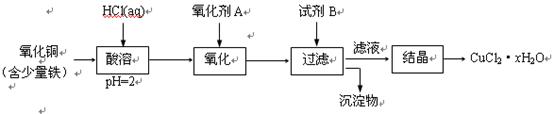
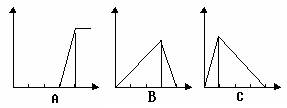
 ����������V����ʱ�䣨t����ϵ��ͼ����Ӧ��þ������
����������V����ʱ�䣨t����ϵ��ͼ����Ӧ��þ������
 + H2O ��C+ NaOH��B+NaCl ��E+ H2O��NaOH+F
+ H2O ��C+ NaOH��B+NaCl ��E+ H2O��NaOH+F ��
��