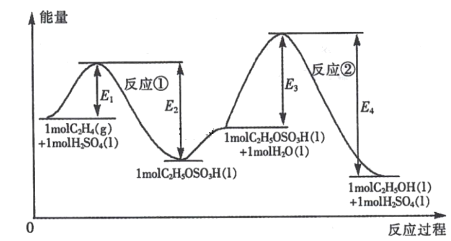
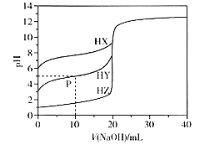
��Ŀ����
����Ŀ������һ�������ĩ�����п��ܺ��������������ʣ�CaCO3��K2CO3��Na2SO4��NaCl��CuSO4���ֽ�������ʵ�飺������ˮ����ɫ��Һ����Һ���������֣�������Һ�м���BaCl2��Һ���ɰ�ɫ�������ټ�����ʱ������ʧ����������ʵ�������ƶϣ�
(1)һ�������ڵ�������_____________________��
(2)д����������ʱ������ʧ�����ӷ�Ӧ����ʽ___________________________��
(3)���ܴ��ڵ�������___________��
(4)д������������ˮ��Һ�еĵ��뷽��ʽ
H2CO3��___________________________��
NH4HCO3 ��___________________________��
Ca(OH)2��___________________________��
���𰸡�CaCO3��Na2SO4��CuSO4 BaCO3+2H+=Ba2++CO2��+H2O NaCl H2CO3![]() H++HCO3- NH4HCO3=NH4++HCO3- Ca(OH)2=Ca2++2OH-
H++HCO3- NH4HCO3=NH4++HCO3- Ca(OH)2=Ca2++2OH-
��������
������ˮ�õ���ɫ������Һ��˵��ԭ����������ɫ�����ʡ�����������������ʡ�������ˮ�����ʣ�
�ڵμӹ�����BaCl2��Һ�����ְ�ɫ��������ɫ����������̼�ᱵ�����ᱵ��̼�ᱵ�������ᣬ���ᱵ���ܣ��ݴ˷�������
��ԭ��Һ��CaCO3������ˮ��CuSO4����ˮ��Ϊ��ɫҺ�壬����ԭ�����ĩ��һ���������������ʣ�
�ڵμӹ�����BaCl2��Һ�����ְ�ɫ��������ɫ����������̼�ᱵ�����ᱵ��̼�ᱵ�������ᣬ���ᱵ���ܣ�������ʱ������ʧ��˵����̼�ᱵ������һ������̼������ӣ�һ����������������ӣ���һ�������ڵ�������CaCO3��Na2SO4��CuSO4��һ����K2CO3�����ܺ���NaCl��
��1��������������֪��һ�������ڵ�������CaCO3��Na2SO4��CuSO4��
��2����������ʱ��̼�ᱵ��ϡ���ᷴӦ�������Ȼ�����ˮ��������̼��̼�ᱵΪ���������֣�HClΪǿ����ʣ���֣��Ȼ���Ϊ�������Σ���֣��������ӷ�Ӧ����ʽΪ��BaCO3+2H+=Ba2��+CO2��+H2O��
��3��������������֪�����ܴ��ڵ������ǣ�NaCl��
��4��H2CO3Ϊ��Ԫ���ᣬ����������ʣ�������Ե�һ��Ϊ��������뷽��ʽΪ��H2CO3![]() H+��HCO3-��NH4HCO3Ϊ�������Σ�����ǿ����ʣ�����뷽��ʽΪ��NH4HCO3=NH4++HCO3-��Ca(OH)2Ϊ������ǿ�����ǿ����ʣ�����뷽��ʽΪ��Ca(OH)2=Ca2++2OH-��
H+��HCO3-��NH4HCO3Ϊ�������Σ�����ǿ����ʣ�����뷽��ʽΪ��NH4HCO3=NH4++HCO3-��Ca(OH)2Ϊ������ǿ�����ǿ����ʣ�����뷽��ʽΪ��Ca(OH)2=Ca2++2OH-��