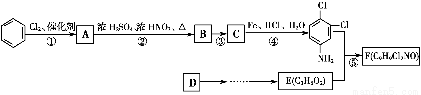
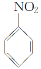
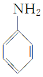
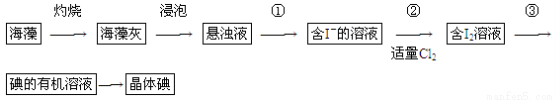
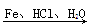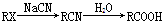ЬтФПФкШн
МиЁЂУОЁЂЗњЁЂХ№ЕШдЊЫидкУПЩ§КЃЫЎжаЕФКЌСПЖМДѓгк1КСПЫЃЌЪєгкКЃЫЎжаЕФГЃСПдЊЫиЁЃ
ЃЈ1ЃЉМиЁЂУОЁЂЗњЁЂХ№ЕчИКадДгДѓЕНаЁЕФХХСаЫГађЪЧ__________________________ЁЃ
ЃЈ2ЃЉяигыХ№ЭЌжїзхЃЌаДГіяидЊЫидзгЕФМлЕчзгХХВМЪНЃЈМДЭтЮЇЕчзгХХВМЪНЃЉЃК________________ЁЃ
ЃЈ3ЃЉгУМлВуЕчзгЖдЛЅГтФЃаЭЭЦЖЯBF3КЭNF3ЕФПеМфЙЙаЭЗжБ№ЮЊ________ЁЂ________ЁЃ
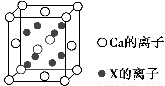
ЃЈ4ЃЉМиЁЂУОЁЂЗњаЮГЩЕФФГЛЏКЯЮяЕФОЇЬхНсЙЙЮЊKЃЋдкСЂЗНОЇАћЕФжааФЃЌMg2ЃЋдкОЇАћЕФ8ИіЖЅНЧЃЌFЃДІгкОЇАћЕФРтБпжааФЁЃгЩМиЁЂУОЁЂЗњаЮГЩЕФИУЛЏКЯЮяЕФЛЏбЇЪНЮЊ________ЃЌУПИіKЃЋгы________ИіFЃХфЮЛЁЃ
ЃЈ1ЃЉFЃОBЃОMgЃОK
ЃЈ2ЃЉ4s24p1
ЃЈ3ЃЉЦНУцШ§НЧаЮЁЁШ§НЧзЖаЮ
ЃЈ4ЃЉKMgF3ЁЁ12
ЁОНтЮіЁПЃЈ1ЃЉЭЌжмЦкжаЃЌЫцзХдзгађЪ§ЕФЕндіЃЌдЊЫиЕчИКаддіДѓЃЛЭЌжїзхжаЃЌЫцзХдзгађЪ§ЕФЕндіЃЌдЊЫиЕчИКадМѕаЁЃЌЖјЧввЛАуЧщПіЯТЃЌЗЧН№ЪєдЊЫиЕФЕчИКадДѓгкН№ЪєдЊЫиЕФЕчИКадЁЃ
ЃЈ2ЃЉяиЮЊ31КХдЊЫиЃЌЮЛгкЕкЫФжмЦкЃЌгыBЭЌжїзхЃЌЙЪзюЭтВуга3ИіЕчзгЃЌМДМлЕчзгХХВМЪНЮЊ4s24p1ЁЃ
ЃЈ3ЃЉBF3ЕФжааФдзгжЛга3ИіМлЕчзгЃЌгы3ИіFдзгЬсЙЉЕФ3ИіЕчзгаЮГЩ3ЖдГЩМќЕчзгЃЌЖјNF3ЕФжааФдзгNга5ИіМлЕчзгЃЌгы3ИіFдзгЬсЙЉЕФ3ИіЕчзгаЮГЩ3ЖдГЩМќЕчзгЃЌЛЙга1ЖдЙТЕчзгЖдЃЌЙЪЧАепЮЊЦНУцШ§НЧаЮЃЌКѓепЮЊШ§НЧзЖаЮЁЃ
ЃЈ4ЃЉОЇАћжаЕФKЃЋЮЊ1ИіЃЌMg2ЃЋЮЊ8ЁС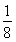 ЃН1ИіЃЌFЃЮЊ12ЁС
ЃН1ИіЃЌFЃЮЊ12ЁС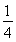 ЃН3ИіЃЌЙЪМиЁЂУОЁЂЗњаЮГЩЕФФГЛЏКЯЮяЕФЛЏбЇЪНЮЊKMgF3ЃЛОЇАћ12ЬѕРтЩЯЕФ12ИіFЃгыДІгкОЇАћжааФЕФKЃЋЕШОрРыЃЌЫљвдУПИіKЃЋгы12ИіFЃХфЮЛЁЃ
ЃН3ИіЃЌЙЪМиЁЂУОЁЂЗњаЮГЩЕФФГЛЏКЯЮяЕФЛЏбЇЪНЮЊKMgF3ЃЛОЇАћ12ЬѕРтЩЯЕФ12ИіFЃгыДІгкОЇАћжааФЕФKЃЋЕШОрРыЃЌЫљвдУПИіKЃЋгы12ИіFЃХфЮЛЁЃ

 ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ
ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ