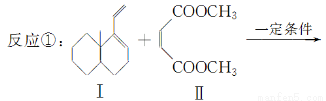
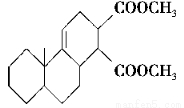
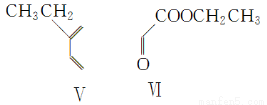��Ŀ����
Ԫ�����ڱ�����A��Ԫ�ذ��������ס��飨As�����ࣨSb���ȡ���ЩԪ���������������Ͳ�����������������ͳ���ʡ�ũҩ�ȷ��涼��������Ҫ�����á���ش��������⣺
��1��N4������һ�ֲ��ȶ��Ķ൪�������������ʷֽ���ܲ������ĵ������ͷų������������ܱ�Ӧ���������ƽ�����ըҩ��N4�����ĸ���ԭ����ɵĵ����������е�ԭ�Ӳ��õĹ���ӻ���ʽΪsp3���÷��ӵĿռ乹��Ϊ________��N��N���ļ���Ϊ________��
��2����̬��ԭ�ӵ����������Ų�ʽΪ________��
��3���縺����������ʾ������ͬԭ���γɻ�ѧ��ʱ�������ϵ������������ǿ������Ԫ�ص�ԭ���ڷ������������õ��Ӷ����������ɴ��ж�N��P��As��Sb�ĵ縺�ԴӴ�С��˳����______________��
��4��������N2H4�����Ա�ʾΪH2N��NH2�����е�ԭ�Ӳ��õĹ���ӻ���ʽΪ________�������ļ��ԱȰ��ļ���________������ǿ����������������ԭ����________________________________________________________________��
д��N2H4��N2O4��Ӧ�Ļ�ѧ����ʽ��____________________��
��5��Ԫ��X��Nͬ��������X��ԭ�Ӱ뾶�Ǹ���������Ԫ��ԭ�Ӱ뾶����С����X��Ca�γɵĻ�����CaX2�ľ����ṹ��ͼ��ʾ��
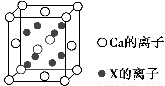
CaX2�ľ���������________��һ�������к���Ca��������Ϊ________������X��������Ϊ________��
��1���������塡60������2��4s24p3
��3��N��P��As��Sb
��4��sp3������N2H4�е�Nԭ���ϵ������ܶ�С��NH3�����ѽ��H����2N2H4��N2O4=3N2��4H2O
��5�����Ӿ��塡4��8
����������1��N4�е�ԭ�ӵĹ���ӻ���ʽΪsp3����ռ乹�Ϳɲ���P4�����ף���ӦΪ��������ṹ��N��N���ļ���Ϊ60������2����λ�ڵ���������A���������������Ų�ʽΪ4s24p3����3��ͬ����Ԫ�ش��ϵ�����Ԫ�صĵ縺����С����4��������HN2��NH2���е�ԭ��������ԭ�ӽ���γ���������������һ�Թµ��Ӷ��������ӻ���ʽΪsp3����NH3�����N2H4����������ΪN2H4�൱��NH3��һ��Hԭ�ӻ�������NH2��Nԭ�ӵ�����������Զǿ��Hԭ�������N2H4�е�Nԭ���ϵ������ܶ�С��NH3�����ѽ��H������5��NԪ��λ�ڵڶ���������������ԭ�Ӱ뾶��С������Ԫ��ΪF��CaF2Ϊ���Ӿ������þ�����Ca2���ĸ���Ϊ8��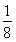 ��6��
��6��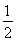 ��4��F�����ھ����ڲ�����Ϊ8����
��4��F�����ھ����ڲ�����Ϊ8����

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�����ͭ���ȷֽ���������ͭ�������������¶Ȳ�ͬ������ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO2��SO3��O2�е�һ�֡����ֻ����֡�ij��ѧ����С��ͨ�����̽����ʵ�����ⶨ��Ӧ������SO2��SO3��O2�����ʵ�����������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ�����������ͼ��ʾ��
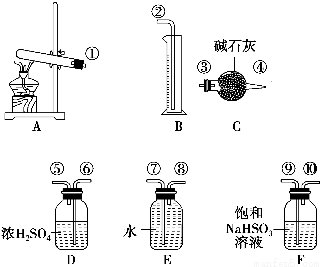
[�������]
��.��������ijɷֿ���ֻ��SO3һ�֣�
��.��������ijɷֿ��ܺ���________���֣�
��.��������ijɷֿ��ܺ���________���֡�
[ʵ��̽��]
ʵ����������ԡ���֪ʵ�����ʱ������ͭ��ȫ�ֽ⡣
��1��������װ̽��ʵ���װ�������������ҵķ������������ӿڵ�����˳��Ϊ��������������������________��________��________��________��������ӿ���ţ���
��2����ʵ�����ʱB����Ͳû���ռ���ˮ����֤������________��ȷ��
��3��������ʵ��С����и�ʵ�������ڼ���ʱ���¶Ȳ�ͬ��ʵ����������������Ҳ��ͬ���������£�
ʵ�� С�� | ��ȡCuSO4 ������/g | װ��C���� ������/g | ��Ͳ��ˮ���������ɱ�״������������/mL |
һ | 6.4 | 2.56 | 448 |
�� | 6.4 | 2.56 | 224 |
��ͨ���������ƶϳ���һС��͵ڶ�С���ʵ��������CuSO4�ֽ�Ļ�ѧ����ʽ��
��һС�飺________________________________________________________��
�ڶ�С�飺________________________________________________________��