��Ŀ����
����Ŀ�������£�0.1 mol/L��H2C2O4��Һ��H2C2O4��HC2O4-��C2O42-��������ռ���ʵ����������ֲ�ϵ������pH�仯�Ĺ�ϵ����ͼ��ʾ�����б�������ȷ����
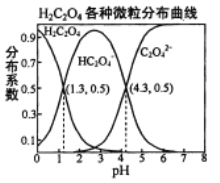
A. HC2O4-![]() H+��C2O42-��K��1��10-4.3
H+��C2O42-��K��1��10-4.3
B. �������ʵ�����NaHC2O4��Na2C2O4����ˮ����������ҺpHǡ��Ϊ4.3
C. ������HF��K��1��10-3.4��������H2C2O4��Һ���뵽����NaF��Һ���������ķ�ӦΪ��H2C2O4��F-��HF��HC2O4-
D. ��0.1 mol/L NaHC2O4��Һ����������Ũ�ȴ�С��ϵΪ��c(Na+)��c(HC2O4-)��c(H+)��c(C2O42-)��c(OH-)
���𰸡�B
��������
A����HC2O4-H++C2O42-����֪K=![]() ��pH=4.3ʱ��c(C2O42-)=c(HC2O4-)������K=c(H+)=1��10-4.3����A��ȷ��B���������ʵ�����NaHC2O4��Na2C2O4����ˮ�У�HC2O4-����̶ȴ���C2O42-��ˮ��̶ȣ�������Һ��c(C2O42-)��c(HC2O4-)������ҺpH����4.3����B����C������ͼ���A�ķ�����������H2C2O4��K1=10-1.3��K2=10-4.3��HF��K=1��10-3.45�������ԣ�H2C2O4��HF��HC2O4-�����Խ�����H2C2O4��Һ���뵽����NaF��Һ�У������ķ�ӦΪH2C2O4+F-=HF+HC2O4-����C��ȷ��D������ͼ��NaHC2O4��Һ�����ԣ���HC2O4-�ĵ���Ϊ��������Һ��HC2O4-���ֵ��룬�������Ũ�ȴ�С��ϵΪ��c(Na+)��c(HC2O4-)��c(H+)��c(C2O42-)��c(OH-)����D��ȷ����ѡB��
��pH=4.3ʱ��c(C2O42-)=c(HC2O4-)������K=c(H+)=1��10-4.3����A��ȷ��B���������ʵ�����NaHC2O4��Na2C2O4����ˮ�У�HC2O4-����̶ȴ���C2O42-��ˮ��̶ȣ�������Һ��c(C2O42-)��c(HC2O4-)������ҺpH����4.3����B����C������ͼ���A�ķ�����������H2C2O4��K1=10-1.3��K2=10-4.3��HF��K=1��10-3.45�������ԣ�H2C2O4��HF��HC2O4-�����Խ�����H2C2O4��Һ���뵽����NaF��Һ�У������ķ�ӦΪH2C2O4+F-=HF+HC2O4-����C��ȷ��D������ͼ��NaHC2O4��Һ�����ԣ���HC2O4-�ĵ���Ϊ��������Һ��HC2O4-���ֵ��룬�������Ũ�ȴ�С��ϵΪ��c(Na+)��c(HC2O4-)��c(H+)��c(C2O42-)��c(OH-)����D��ȷ����ѡB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�