��Ŀ����
A��ԭ������С��36��X��Y��Z��W����Ԫ�أ�����X���γɻ�����������Ԫ�أ�Yԭ�ӻ�̬ʱ���������������ڲ��������2����Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�W��ԭ������Ϊ29��
�ش��������⣺
��1�� ������Yԭ�ӹ�����ӻ�����Ϊ
��1mol
������Yԭ�ӹ�����ӻ�����Ϊ
��1mol ����
���� ������ĿΪ ��
������ĿΪ ��
��2�������� �ķе�Ȼ�����
�ķе�Ȼ����� �ĸߣ�����Ҫԭ����
��
�ĸߣ�����Ҫԭ����
��
��3��Ԫ��Y��һ����������Ԫ��Z��һ�������ﻥΪ�ȵ����壬Ԫ��Z������������ķ���ʽ�� ��
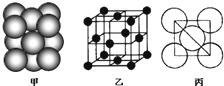
��4��Ԫ��W��һ���Ȼ��ᄃ��ľ����ṹ��ͼ13��ʾ�����Ȼ���Ļ�ѧʽ��
��������Ũ���ᷢ����������ԭ��Ӧ����������� ����Ӧ�Ļ�ѧ����ʽΪ
��
����Ӧ�Ļ�ѧ����ʽΪ
��

B.������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ14��ʾ�����ȡ�����������̶�װ�þ�����ȥ��

ʵ��������£�
�ٽ�һ�����ĵ���ˮ��Һ��������ƿ��
�ڿ��Ʒ�ӦҺ�¶���55��60�������£��߽�������μ�һ�����������������Ļ��ᣨ65%HNO3��98%H2SO4��������Ϊ2��1.5����Һ
�۷�Ӧ3h���ң���ȴ�����˺����ؽᾧ�ò��ᾧ�塣
������������ˮ��Һ�����пɷ������з�Ӧ��
C6H12O6+12HNO3 ��3H2C2O4+9NO2�� +3NO�� +9H2O
C6H12O6+8HNO3 ��6CO2+8NO�� +10H2O
3H2C2O4+2HNO3 ��6CO2+2NO�� +4H2O
(1)��������Ƿ�ˮ����ȫ�����õ��Լ�Ϊ
(2)ʵ����������μӹ��죬�����²�������½�����ԭ����
(3)װ��C����β�����գ���β����n(NO2):n(NO)=1��1ʱ��������NaOH��Һ�ܽ�NO��ȫ�����գ�ԭ���� ���û�ѧ����ʽ��ʾ��
��4������NaOH��Һ����β����Ƚϣ����õ���ˮ��Һ����β�������š�ȱ���� ��
��5�������ؽᾧ�ļ�ѹ���˲����У����ձ����������⣬������ʹ�����ڹ����β��ϵ������� ��
A. ��1��sp�ӻ� 3mol��3��6.2��10 ��
��2��NH ���Ӵ������
��3��N O
O
��4��CuCl CuCI+2HCI=====H2CuCI3 (��CuCI+2HCI===H2[CuCI3])
B. (1) ��ˮ��KI-I2 ��Һ
(2)�����¶ȹ��ߡ�����Ũ�ȹ�����C6H12O6 ��H2C2O4��һ��������
(3)NO2+NO+2NaNO2+H2O
(4)�ŵ㣺���HNO3������ ȱ�㣺NO2���ղ���ȫ
(5)����©��������ƿ
����������

 ��2012?��ͨģ�⣩��˳������������B Rosenberg������1969�귢�ֵĵ�һ�־��п������ԵĽ����������Ļ�ѧʽΪPt��NH3��2Cl2��1995��WHO���ϰ����ΰ�ҩ�����������˳�����ۺ������е�2λ��
��2012?��ͨģ�⣩��˳������������B Rosenberg������1969�귢�ֵĵ�һ�־��п������ԵĽ����������Ļ�ѧʽΪPt��NH3��2Cl2��1995��WHO���ϰ����ΰ�ҩ�����������˳�����ۺ������е�2λ��
 ������Yԭ�ӹ�����ӻ�����Ϊ ��1mol
������Yԭ�ӹ�����ӻ�����Ϊ ��1mol ������ĿΪ ��
������ĿΪ �� �ķе�Ȼ�����
�ķе�Ȼ����� �ĸߣ�����Ҫԭ����
�ĸߣ�����Ҫԭ����  ��
�� ����Ӧ�Ļ�ѧ����ʽΪ ��
����Ӧ�Ļ�ѧ����ʽΪ ��
