��Ŀ����
����˵����ȷ����
| A��NaHCO3��Na2CO3�����Һ�У�һ����c(Na+)+c(H+)��c(OH-)+c(HCO3-)+c(CO32-) |
| B��Ũ�Ⱦ�Ϊ0.1mol��L-1��������Һ��pH�ɴ�С����˳��ΪNaOH��Na2CO3��NaHSO4��(NH4)2SO4 |
| C��pH��3�������������Һ��c(SO42��)��c(CH3COO��)֮��Ϊ1:2 |
| D�������������μ�ˮ����Һ�ĵ����ԡ�����ĵ���̶ȡ�pH����������С |
C
�������������A�����ݵ���غ��֪��NaHCO3��Na2CO3�����Һ�У�һ����c(Na��)��c(H��)��c(OH��)��c(HCO3��)��2c(CO32��)��A����ȷ��B������������ǿ���Һ�Լ��ԡ�̼������ǿ�������Σ�CO32��ˮ�⣬��Һ�Լ��ԡ�����������ǿ�����ʽ�Σ�����������ӣ���Һ�����ԡ��������ǿ�������Σ�NH4��ˮ�⣬��Һ�����ԣ���������ҺŨ����ͬ�������£�pH�ɴ�С����˳��ΪNaOH��Na2CO3��(NH4)2SO4��NaHSO4��B����ȷ��C�����ݵ���غ��֪���ڴ����������Һ�зֱ����c(H+)��c(OH��)��c(CH3COO��)��c(H+)��c(OH��)��2c(SO42��)��������Һ��pH��3��������Һ��c(SO42��)��c(CH3COO��)֮��Ϊ1:2��C��ȷ��D�����������ᣬ���ڵ���ƽ�⣬ϡ�ʹٽ����룬��������������μ�ˮ������ĵ���̶�һֱ������Һ�ĵ����ԡ�����������С������Һ��pH���ȼ�С������D����ȷ����ѡC��
���㣺��������ˮ�⡢������ʵĵ��롢��Һ������Ũ�ȴ�С�Ƚ��Լ���Һ����Ժ͵����Ե��жϵ�

�����£���0.10mol/L������ζ�20.00mL 0.10mol/L��ij��BOH��Һ�õ��ĵζ��������£������жϲ���ȷ����( )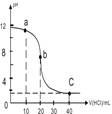
| A��a��ʱ����Һ�ʼ��ԣ���Һ��c(B+)>c(Cl-) |
| B��b��ʱ��Һ��pH=7 |
| C����c(Cl-)=c(B+)ʱ��V(HCl)<20mL |
| D��C��ʱ��Һ��c(H+)ԼΪ0.03mol/L |
��ҵ����Na2CO3��Һ���ݹ�¯ˮ��ʹ����CaSO4��ת��ΪCaCO3�������й�CaSO4��CaCO3�����й��Ʋ���ȷ����
| A��CaSO4��һ��ǿ�������� |
| B��CaSO4�ܽ��С��CaCO3 |
| C��CaSO4�ܽ�ȴ���CaCO3 |
| D��CaSO4�ܽ�ȵ���CaCO3 |
�����£�0.1mol/L HX��pH=1��0.1 mol/LCH3COOH��pH=2.9������˵���в���ȷ����
| A��HX��CH3COOH��������ˮ�ĵ��� |
| B����HCl��HX��0.1mol����ˮ���1L�����Һ������Һ��c(H+)="0.2" mol/L |
| C�������ʵ���Ũ�ȵ������HX��CH3COONa����Һ��Ϻ�������Һ�У� c(Na+)��c(CH3COOH)��c(CH3COO-)��c(H+)��c(OH-) |
| D�����Ũ�ȵ������HX��CH3COOH��Һ�У��ֱ����ͬŨ�ȵģ�aOH��Һ����ʹ����pH������7������������������Һ�����ǰ�ߴ��ں��� |
��ѧ�г�����ͼ������ʾij�ֱ仯���̣����й���4��ͼ���˵����ȷ����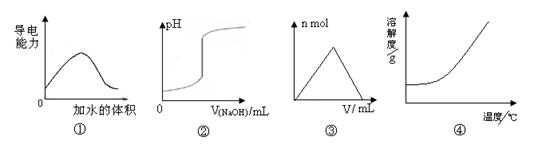
| A��ͼ�ٿɱ�ʾt��ʱ��������ϡ��������Һ�����Եı仯 |
| B��ͼ�ڿɱ�ʾ��һ���������еμ�һ��Ũ������������Һʱ����ҺpH�ı仯 |
| C��ͼ�ۿɱ�ʾ��һ����������Һ�еμ�һ��Ũ������������Һʱ���������������ʵ����ı仯 |
| D��ͼ�ܿɱ�ʾ���еĹ��������ܽ�����¶ȵı仯 |
����˵���������ȷ����
| A����ˮ���������c(H+)��1��10��2mol?L��1��Һ�У����ܴ���:CO32����NH4+��Cl-��Na+ |
| B��ij����ϡ��Һ��pH��a��������Һϡ��1������Һ��pH��b����a��b |
| C��Ba(OH)2��Һ�м��˹�����Al2(SO4)3��Һ�������ӷ���ʽΪ�� 3Ba2++6OH- +2Al3++3SO42-��3BaS04��+2Al(OH)3�� |
| D�����ʵ���Ũ�Ⱦ�Ϊ1 mol?L��1��NaCl��MgCl2���Һ�У�����Cl-����ĿΪ3NA(NA��ʾ�����ӵ�������ֵ�� |
25��ʱ��CaCO3��ˮ�е��ܽ�ƽ��������ͬ��ʾ����֪25��ʱ��CaCO3��Ksp=2.8��10-9����ͼ����������˵������ȷ����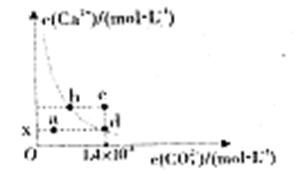
| A��x����ֵΪ2��10-5 |
| B��c��ʱ��̼��Ƴ������� |
| C��b����d���Ӧ���ܶȻ���� |
| D����������ˮ��ʹ��Һ��d��䵽a�� |
��˫ѡ��������Һ����Ũ�ȹ�ϵһ����ȷ����
| A�������£���ˮ���Ȼ�淋�pH=7�Ļ����Һ�У�c(Cl��)=c(NH4+) |
| B��pH=1��һԪ���pH=13��һԪ��������ϣ�c(OH��)=c(H+) |
| C��0.1 mol��L��1���������Һ�У�c(NH4+)>c(SO42��)>c(H+)> c(OH��) |
| D��0.1 mol��L��1��������Һ�У�c(OH��)=c(H+)+c(HS��)+c(H2S) |