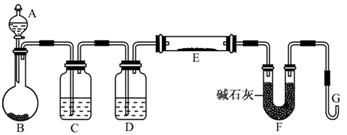
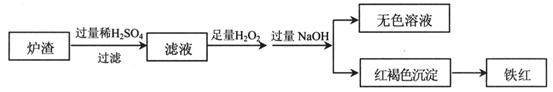
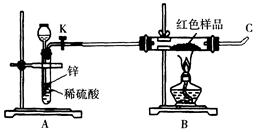
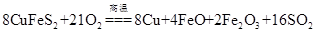��Ŀ����
Ϊ̽��Fe(NO3)2���������ȷֽ����Ͳ�������ʣ�ij��ѧС�鿪չ����̽����
���������ϡ�2KNO3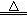 2KNO2+O2�� Fe(NO3)2
2KNO2+O2�� Fe(NO3)2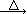 FexOy+NO2��+O2��
FexOy+NO2��+O2��
ʵ��һ��̽��Fe(NO3)2�ȷֽ�����������Ԫ�صļ�̬����С���ͬѧ���ֽ��Ĺ����������������ϡH2SO4�õ���Ӧ������Һ����������̽��ʵ�顣
��1�����ᴿ���롿
����һ����Ԫ��ֻ��+2�ۣ�
���������Ԫ�� ��
����������Ԫ�ؼ���+2������+3�ۡ�
��ʵ�����������һ����Һ�е���KSCN��Һ������һ����Һ�е�������KMnO4ϡ��Һ��
��2����ʵ������ʵ��� ��ʵ��� ��
��3����ʵ����ۡ��������������Fe(NO3)2�ֽ�Ļ�ѧ����ʽ�� ��
ʵ�����
��4��̽��Fe(NO3)2�ȷֽ������������ʡ�С����ͬѧ����������ʵ�飬�����ʵ���ȱ�������ݡ���ѡ�Լ�����Ʒ��ŨH2SO4��Һ��4mol/LNaOH��Һ��0.1mol/LBaCl2��Һ�������ǵ�ľ����0.1mol/L����KMnO4��Һ������ˮ��
ʵ������KNO3�л���Fe(NO3)2��Ϊȷ��������Ԫ�صĺ�����С���ͬѧ��������ʵ�飺��ȡ�������Ʒ10g����ּ��ȷֽ⣻�ڽ���������ܽ⡢���ˣ�ȡ��������ϴ�ӡ�����Ƶ�������Ϊ3.2g������������Ԫ�ص���������Ϊ ����������λ��Ч���֣����ԭ��������Fe-56 O-16��
���������ϡ�2KNO3
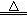 2KNO2+O2�� Fe(NO3)2
2KNO2+O2�� Fe(NO3)2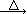 FexOy+NO2��+O2��
FexOy+NO2��+O2��ʵ��һ��̽��Fe(NO3)2�ȷֽ�����������Ԫ�صļ�̬����С���ͬѧ���ֽ��Ĺ����������������ϡH2SO4�õ���Ӧ������Һ����������̽��ʵ�顣
��1�����ᴿ���롿
����һ����Ԫ��ֻ��+2�ۣ�
���������Ԫ�� ��
����������Ԫ�ؼ���+2������+3�ۡ�
��ʵ�����������һ����Һ�е���KSCN��Һ������һ����Һ�е�������KMnO4ϡ��Һ��
��2����ʵ������ʵ��� ��ʵ��� ��
��3����ʵ����ۡ��������������Fe(NO3)2�ֽ�Ļ�ѧ����ʽ�� ��
ʵ�����
��4��̽��Fe(NO3)2�ȷֽ������������ʡ�С����ͬѧ����������ʵ�飬�����ʵ���ȱ�������ݡ���ѡ�Լ�����Ʒ��ŨH2SO4��Һ��4mol/LNaOH��Һ��0.1mol/LBaCl2��Һ�������ǵ�ľ����0.1mol/L����KMnO4��Һ������ˮ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����Fe(NO3)2�������Թ��У����ȷֽ⡣ | ��˵���ֽ�����������к���NO2�� |
| ����2������������������ͨ��ʢ������ ��Ũ�����ϴ��ƿ�� �����һ�����ڼ��顣 | ��˵���ֽ�����������к�O2�� |
ʵ������KNO3�л���Fe(NO3)2��Ϊȷ��������Ԫ�صĺ�����С���ͬѧ��������ʵ�飺��ȡ�������Ʒ10g����ּ��ȷֽ⣻�ڽ���������ܽ⡢���ˣ�ȡ��������ϴ�ӡ�����Ƶ�������Ϊ3.2g������������Ԫ�ص���������Ϊ ����������λ��Ч���֣����ԭ��������Fe-56 O-16��
��17�֣���1��ֻ��+3�ۣ�1�֣�
��2����Һ����Ѫ��ɫ��2�֣�������������Ҳ���֣� ��Һ�Ϻ�ɫ����ɫ��2�֣�
��3��4Fe(NO3)2 2Fe2O3+8NO2��+O2����2�֣���ѧʽ�������֣�ϵ������1�֣���д������1�֣�
2Fe2O3+8NO2��+O2����2�֣���ѧʽ�������֣�ϵ������1�֣���д������1�֣�
��4��
��5��22.4%
��2����Һ����Ѫ��ɫ��2�֣�������������Ҳ���֣� ��Һ�Ϻ�ɫ����ɫ��2�֣�
��3��4Fe(NO3)2
 2Fe2O3+8NO2��+O2����2�֣���ѧʽ�������֣�ϵ������1�֣���д������1�֣�
2Fe2O3+8NO2��+O2����2�֣���ѧʽ�������֣�ϵ������1�֣���д������1�֣���4��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����Fe(NO3)2�������Թ��У����ȷֽ⡣ | �к���ɫ���������2�֣�������������Ҳ���֣���˵���ֽ�����������к���NO2�� |
| ����2������������������ͨ��ʢ������ 4mol/LNaOH��Һ��2�֣���Ũ�����ϴ��ƿ���ô����ǵ�ľ����2�֣������һ�����ڼ��顣 | �����ǵ�ľ����ȼ��1�֣���˵���ֽ�����������к�O2�� |
��5��22.4%
�����������1������������֪�IJ����ƶϣ��������Ϊ��Ԫ��ֻ��+3�ۣ���2������ʵ������ƶϣ�Fe(NO3)2���ȷֽ�����Ĺ������ֻ��Fe2O3��Fe2O3������ϡ���ᷴӦ����Fe3+����Һ����Fe3+��KSCN��Һ��죬����ʹ���Ը��������Һ��ɫ����Fe2O3��ϵ��Ϊ1����������������ԭ�Ӹ����غ���ƽ���÷�ӦΪ2Fe(NO3)2
 Fe2O3+4NO2��+1/2O2����ϵ���ӱ��ɵã�4Fe(NO3)2
Fe2O3+4NO2��+1/2O2����ϵ���ӱ��ɵã�4Fe(NO3)2 2Fe2O3+8NO2��+O2������4��Fe(NO3)2���ȷֽ��������������У�NO2�Ǻ���ɫ���壬O2����ɫ��ζ���壬�к���ɫ�������������˵���ֽ�����������к���NO2������NO2�ж���������ˮ��Ӧ��������O2��Ӧ��NO����˲���2��Ӧ�����ù���4mol/LNaOH��Һ���ն����NO2������Ũ�����������һ���������ô����ǵ�ľ�����飬��������ľ����ȼ��˵���ֽ�����������к���O2����5�����������֪��������m(Fe2O3)=3.2g��������Ԫ���غ�ɵù�ϵʽ��2Fe��Fe2O3����ԭ����m(Fe)= m(Fe2O3)��2��56/160=3.2g��2��56/160�����ڻ����������Ϊ10g������������Ԫ�ص���������Ϊ3.2g��2��56/160��10g��100%=22.4%��
2Fe2O3+8NO2��+O2������4��Fe(NO3)2���ȷֽ��������������У�NO2�Ǻ���ɫ���壬O2����ɫ��ζ���壬�к���ɫ�������������˵���ֽ�����������к���NO2������NO2�ж���������ˮ��Ӧ��������O2��Ӧ��NO����˲���2��Ӧ�����ù���4mol/LNaOH��Һ���ն����NO2������Ũ�����������һ���������ô����ǵ�ľ�����飬��������ľ����ȼ��˵���ֽ�����������к���O2����5�����������֪��������m(Fe2O3)=3.2g��������Ԫ���غ�ɵù�ϵʽ��2Fe��Fe2O3����ԭ����m(Fe)= m(Fe2O3)��2��56/160=3.2g��2��56/160�����ڻ����������Ϊ10g������������Ԫ�ص���������Ϊ3.2g��2��56/160��10g��100%=22.4%��
��ϰ��ϵ�д�
�����Ŀ
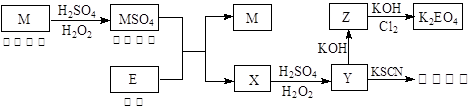
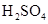 ��
��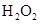 ���Һ�����ӷ���ʽ�� ��
���Һ�����ӷ���ʽ�� ��