��Ŀ����
��ͭ��(CuFeS2)����ȡͭ���仯�������Ҫԭ��֮һ������¯������Ҫ�ɷ� ��FeO��Fe2O3��SiO2��Al2O3��������������ת����ϵ,��ش�

��1��д����֤��SO2�������������������ԵĻ�ѧ����ʽ________________��
��2����NaOH��Һ����SO2����NaHSO3��ҺpH��7�������Һ�д������ӵ����ʵ���Ũ���ɴ�С��˳����________��
��3��д��Cu2S������ȡ��ͭ�Ļ�ѧ����ʽ________________________
��4���ϵ��Һ�г�����Pb2+��Zn2+����ϵ��Һ�м���Na2S��Һ������PbS��ZnS����ʱ��C(Zn2+): C(Pb2+)��________��[��֪��Ksp(PbS)��3.4��10��28mol2��L��2��Ksp(ZnS)��1.6��10��24mol2��L��2��]
��5��д��֤����ҺI�к���Fe2+��ʵ�����________________��
��6��Na2FeO4��ɱ����ˮ��ԭ����________________��
��7��Na2FeO4��Zn������ɼ��Ե�أ��䷴ӦʽΪ:3Zn+2FeO42-+8H2O��3Zn(OH)2+2Fe(OH)+4OH-����д���ŵ�ʱ�����缫��Ӧʽ________________��

��1��д����֤��SO2�������������������ԵĻ�ѧ����ʽ________________��
��2����NaOH��Һ����SO2����NaHSO3��ҺpH��7�������Һ�д������ӵ����ʵ���Ũ���ɴ�С��˳����________��
��3��д��Cu2S������ȡ��ͭ�Ļ�ѧ����ʽ________________________
��4���ϵ��Һ�г�����Pb2+��Zn2+����ϵ��Һ�м���Na2S��Һ������PbS��ZnS����ʱ��C(Zn2+): C(Pb2+)��________��[��֪��Ksp(PbS)��3.4��10��28mol2��L��2��Ksp(ZnS)��1.6��10��24mol2��L��2��]
��5��д��֤����ҺI�к���Fe2+��ʵ�����________________��
��6��Na2FeO4��ɱ����ˮ��ԭ����________________��
��7��Na2FeO4��Zn������ɼ��Ե�أ��䷴ӦʽΪ:3Zn+2FeO42-+8H2O��3Zn(OH)2+2Fe(OH)+4OH-����д���ŵ�ʱ�����缫��Ӧʽ________________��
��ÿ��2�֣���14�֣���1��SO2+2H2S��3S��+2H2O ��2��c(Na+)��c(HSO3-)��c(H+)��c(SO32-)��c(OH-)
��3��Cu2S+O2 2Cu+SO2 ��4��
2Cu+SO2 ��4��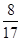 ��104
��104
��5��ȡ��ҺI�������Թ��У�Ȼ��μ���������KMnO4��Һ���Ϻ�ɫ��ȥ��֤����Fe2+��
��6��Na2FeO4��ǿ��������ɱ������ˮ�б��Fe3+��Fe3+ˮ������Fe(OH)3�����ܾ�ˮ����ÿ��Ҫ���ռ1�֣���2�֣� ��7��2FeO42-+6e��+8H2O��2Fe(OH)3+10OH��
��3��Cu2S+O2
 2Cu+SO2 ��4��
2Cu+SO2 ��4��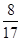 ��104
��104 ��5��ȡ��ҺI�������Թ��У�Ȼ��μ���������KMnO4��Һ���Ϻ�ɫ��ȥ��֤����Fe2+��
��6��Na2FeO4��ǿ��������ɱ������ˮ�б��Fe3+��Fe3+ˮ������Fe(OH)3�����ܾ�ˮ����ÿ��Ҫ���ռ1�֣���2�֣� ��7��2FeO42-+6e��+8H2O��2Fe(OH)3+10OH��
�����������1���������ڷ�Ӧ�еõ����ӣ����ϼ۽��͡����������������ˮ��Һ������Ӧ���ɵ����������SO2���������������������ԣ���Ӧ�Ļ�ѧ����ʽ��SO2+2H2S��3S��+2H2O��
��2��NaHSO3��Һ������������������۵��뻹��ˮ�ⶼ�ǽ����ģ�NaHSO3��Һ�����ԣ�˵��������������ӵ�ˮ��̶�С�������̶ȣ������Һ������Ũ�ȴ�С˳����c(Na+)��c(HSO3-)��c(H+)��c(SO32-)��c(OH-)��
��3����ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ���ڷ�Ӧ��ͭԪ�ػ��ϼ��ɣ�1�۽��͵�0�ۣ��õ�1�����ӡ���Ԫ�ػ��ϼ���0�۽��͵���2�ۣ��õ�2�����ӡ���Ԫ�ػ��ϼ��ɣ�2�����ߵ���4�ۣ�ʧȥ6�����ӡ���˸��ݵ��ӵĵ�ʧ�غ��֪������Ӧ��������ԭ��ӦΪCu2S+O2
 2Cu+SO2��
2Cu+SO2����4������PbS��ZnS����ʱ����Һ��c(S2��)��ͬ������Һ��
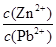 =
=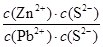 ��
��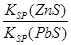 ��
��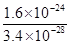 ��
��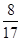 ��104��
��104����5���������Ӿ��л�ԭ�ԣ���ʹ���Ը��������Һ��ɫ���ݴ˿��Լ����������ӡ������ȷ�IJ�����ȡ��ҺI�������Թ��У�Ȼ��μ���������KMnO4��Һ���Ϻ�ɫ��ȥ��֤����Fe2+��
��6��Na2FeO4�ǿ�����ˮ��ǿ����������ˮ����ɱ�����������ã��仹ԭ������Fe3+��������ˮ������Fe(OH)3���壬Fe(OH)3����������ˮ�е������Ĺ���������Ӷ��ﵽ��ˮ��Ŀ�ġ�
��7��ԭ����и���ʧȥ���ӣ�����������Ӧ�������õ����ӣ�������ԭ��Ӧ�����Ը����ܷ�Ӧʽ��֪��FeO42-�õ����ӣ��������������������缫��ӦʽΪ��FeO42-+3e��+4H2O��Fe(OH)3+5OH����

��ϰ��ϵ�д�
 ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ
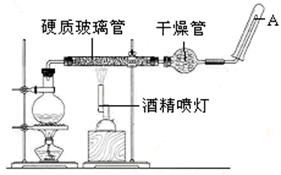



 2KNO2+O2�� Fe(NO3)2
2KNO2+O2�� Fe(NO3)2 FexOy+NO2��+O2��
FexOy+NO2��+O2��