��Ŀ����
����Ŀ������(CH3OCH3)��һ��������Դ��
��֪��CO(g)+2H2(g)CH3OH(g) H1=-99kJ/mol
��2CH3OH(g)CH3OCH3(g)+H2O(g) H2=-24kJ/mol
��CO(g)+H2O(g)CO2(g)+H2(g) H3=-41kJ/mol
�ش��������⣺
(1)д��CO��H2��Ӧ����CO2��CH3OCH3(g)���Ȼ�ѧ����ʽ��________________��
(2)���д�ʩ����߷�Ӧ����CO��ƽ��ת���ʵ���________(����ĸ)��
A.����ѹǿ B.�����¶� C.����H2Ũ�� D.�Ӹ�Ч����
(3)�ں��º���������ֻ������Ӧ�ۣ�������������÷�Ӧ�ﵽƽ�����________(����ĸ)��
A.����ѹǿ���ֲ��� B.�����ܶȱ��ֲ���
C.![]() ���ֲ��� D.Ũ���̱��ֲ���
���ֲ��� D.Ũ���̱��ֲ���
(4)��һ���¶�(T��)�£�������ܱ�������Ͷ��һ����CH3OH���壬ֻ������Ӧ�ڡ�����������CH3OCH3�����ʵ�������[��(CH3OCH3)]�뷴Ӧʱ��(t)���й����������ʾ��
t/min | 0 | 15 | 30 | 45 | 80 | 100 |
[��(CH3OCH3)] | 0 | 0.05 | 0.08 | 0.09 | 0.10 | 0.10 |
��30minʱ��CH3OH��ת������(CH3OH)________�������¶��£�������Ӧ��ƽ�ⳣ��K=________���÷�����ʾ����
�ڷ�Ӧ����v=v��-v��������v��=k����2(CH3OH)��v��=k����(CH3OCH3)��(H2O)��k����k���ֱ�Ϊ�����淴Ӧ���ʳ�����ֻ���¶��йأ���Ϊ���ʵ���������15minʱ![]() ________(�������2λС��)
________(�������2λС��)
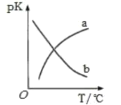
(5)���ܱ������з�����Ӧ�ۣ�ƽ�ⳣ��ΪK��pK=-lg2K��pK�¶ȵĹ�ϵ��ͼ��ʾ��ͼ������________������a������b�����ܷ�ӳƽ�ⳣ���仯���ơ�
(6)��CO2�������ı���KHCO3��Һ�У������CO2�Ʊ�CH3OCH3��ԭ����ͼ��ʾ��������HCO3-���ɣ��õ�ĵ缫��ӦʽΪ________��
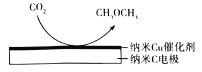
���𰸡�3CO(g)+3H2(g)CO2(g)+CH3OCH3(g) H=-263kJ/mol AB CD 16 ![]() 5.06 a 14CO2+12e-+9H2O=CH3COH3+12HCO3-
5.06 a 14CO2+12e-+9H2O=CH3COH3+12HCO3-
��������
(1)���ݸ�˹����2��+��+���ɵ�����
(2)������������ԭ���жϡ�
(3)��Ӧ��������ļ���������������ȣ���Ӧ��ʼ���գ�������ѹǿ�����ʵ������ܶȲ��䣬������Ϊƽ��ı�־����Ӧƽ��ʱ��n(CO)��n(CO2)�������ٸı䣬Ũ�Ȳ��ٸı䣻
(4)���ݱ������ݣ���Ӧ��80minʱ�ﵽƽ��״̬����(CH3OCH3)Ϊ0.10��
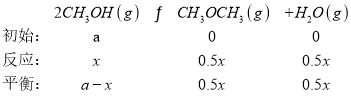
(5)��Ӧ�����ʱ�С���㣬Ϊ���ȷ�Ӧ�������¶�ƽ�������ƶ���ƽ�ⳣ����С��
(6)��������õ�����CO2����CH3OCH3ʱ����HCO3-���ɡ�
(1)��3CO(g)+3H2(g)CO2(g)+CH3OCH3(g)�����ݸ�˹����2��+��+���ɵ�����3CO(g)+3H2(g)CO2(g)+CH3OCH3(g) H=-263kJ/mol��
(2)��߷�Ӧ����CO��ƽ��ת���ʣ���ƽ�������ƶ���������������ԭ������ѹǿ�ͽ����¶ȿ�ʹƽ�������ƶ��������������������ΪAB��
(3)��Ӧ��������ļ���������������ȣ���Ӧ��ʼ���գ�������ѹǿ�����ʵ������ܶȲ��䣬������Ϊƽ��ı�־����Ӧƽ��ʱ��n(CO)��n(CO2)�������ٸı䣬Ũ�Ȳ��ٸı䣬��ΪCD��
(4)���ݱ������ݣ���Ӧ��80minʱ�ﵽƽ��״̬����(CH3OCH3)Ϊ0.10��
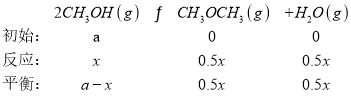
��30minʱ����������ʽ��![]() =0.08�����x=0.16a��CH3OH��ת������(CH3OH)=
=0.08�����x=0.16a��CH3OH��ת������(CH3OH)=![]() ��100%=16%��ͬ����ƽ��ʱx=0.2��K=
��100%=16%��ͬ����ƽ��ʱx=0.2��K=![]() =
=![]() ��
��
��15minʱ����������ʽ��![]() =0.05�����x=0.1a������(CH3OCH3)=��(H2O)=0.05a����2(CH3OH)=(0.9a)2��ƽ��ʱ��v��=k����2(CH3OH)=v��=k����(CH3OCH3)��(H2O)��
=0.05�����x=0.1a������(CH3OCH3)=��(H2O)=0.05a����2(CH3OH)=(0.9a)2��ƽ��ʱ��v��=k����2(CH3OH)=v��=k����(CH3OCH3)��(H2O)��![]() =K��
=K��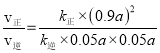 =
=![]() =5.06��
=5.06��
(5)��Ӧ�����ʱ�С���㣬Ϊ���ȷ�Ӧ�������¶�ƽ�������ƶ���ƽ�ⳣ����С��pK=-lg2K����pK����a�ܷ�ӳƽ�ⳣ���仯���ƣ�
(6)��CO2�������ı���KHCO3��Һ�У���������õ��ӣ���CO2����CH3OCH3ʱ����HCO3-���ɣ��缫��ӦʽΪ14CO2+12e-+9H2O=CH3COH3+12HCO3-��

 53���ò�ϵ�д�
53���ò�ϵ�д�����Ŀ��1Lij��Һ�к��е��������±���
���� |
|
|
|
|
���ʵ���Ũ��
| 1 | 1 |
| 1 |
�ö��Ե缫������Һ������·����![]() ͨ��ʱ�����Ե��ʱ��Һ����ı仯���缫������ܴ��ڵ��ܽ���������˵����ȷ����
ͨ��ʱ�����Ե��ʱ��Һ����ı仯���缫������ܴ��ڵ��ܽ���������˵����ȷ����
A.��������Һ��pH=0B.a=3
C.��������![]() D.���������Ľ�����ͭ����
D.���������Ľ�����ͭ����