��Ŀ����
9����֪A��B��֧�Թ���ʢ����Һ�й�����K+��Ag+��Mg2+��Cl-��OH-��NO3-�������ӣ����Թ�A����Һ�е����̪��Һ�ʷۺ�ɫ����ش��������⣺��1���Թ�A����Һ������������������4�֣�
��2������ij�Թ��е���ϡ�����������������Թ�ΪB���A����B������
��3�������Թ�A���Թ�B�е���Һ��һ������Ȼ�Ϲ��˺õ���Һ����һ�����ʵ���Һ�����Ϲ����з�����Ӧ�����ӷ���ʽΪAg++Cl-�TAgCl����Mg2++2OH-�TMg��OH��2����
��4���������Թ�A��Һ�е���������ɵ�̼��������Һ�У���������Ba��OH��2��Һ��������Ӧ�����ӷ���ʽΪ2HCO3-+Ba2++2OH-�TBaCO3��+2H2O+CO32-��
���� �Թ�A����Һ�е����̪��Һ�ʷۺ�ɫ��˵����Һ�Լ��ԣ�һ������OH-����Aһ��û��Ag+��Mg2+��Ӧ����K+���Թ�B�к���Ag+��Mg2+����һ��û��Cl-��Ӧ����NO3-����϶�Ӧ���ӵ����ʽ����⣮
��� �⣺�Թ�A����Һ�е����̪��Һ�ʷۺ�ɫ��˵����Һ�Լ��ԣ�һ������OH-����Aһ��û��Ag+��Mg2+��Ӧ����K+���Թ�B�к���Ag+��Mg2+����һ��û��Cl-��Ӧ����NO3-��
��1���Թ�A����Һ�к���K+��OH-��Cl-�����ܺ���NO3-�����4�֣��ʴ�Ϊ��4��
��2������ij�Թ��е���ϡ�����������������Թ��к���Ag+��Ϊ�Թ�B���ʴ�Ϊ��B��
��3�������Թ�A���Թ�B�е���Һ��һ������Ȼ�Ϲ��˺�������Һ�ɵõ�һ�ִ������Ag+��Cl-ǡ����ȫ��Ӧ��Mg2+��OH-ǡ����ȫ��Ӧ��ʣ������Ӻ���������ӣ���Ϲ����з�����Ӧ�����ӷ���ʽΪ��Ag ++Cl-=AgCl����Mg2++2OH-=Mg��OH��2����
�ʴ�Ϊ��Ag++Cl-�TAgCl����Mg2++2OH-�TMg��OH��2����
��4����KHCO3��Һ�У���������Ba��OH��2��Һ��������Ӧ�����ӷ���ʽΪ2HCO3-+Ba2++2OH-�TBaCO3��+2H2O+CO32-���ʴ�Ϊ��2HCO3-+Ba2++2OH-�TBaCO3��+2H2O+CO32-��
���� ���⿼�������ӹ��漰���ӷ���ʽ����д��Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬��Ŀ�Ѷ��еȣ������漰�����ݽ϶࣬��ֿ�����ѧ������ѧ֪ʶ�����������ע���������ӷ�Ӧ���������������ӷ���ʽ����д������

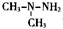 �����ػ��������ȼ�ϣ�C2H8N2�����к�̼̼������ͬ���칹�干�У�������
�����ػ��������ȼ�ϣ�C2H8N2�����к�̼̼������ͬ���칹�干�У�������| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
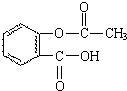 ��ѧ�ͻ���������ʳƷӪ��������Ӧ�õ�������أ�
��ѧ�ͻ���������ʳƷӪ��������Ӧ�õ�������أ� ��
��
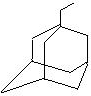 �����������ʾΪR-CH2CH3����
�����������ʾΪR-CH2CH3����
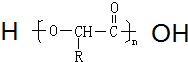 ��
��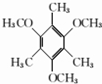 ��
�� ��Ũ������Ũ�����Ϲ����л�������������嵥��X��������M����ɫҺ�壬������M������ԭ�Ӿ��ﵽ8���ӵ��ȶ��ṹ��2molM���ȷֽ�����lmol X��2mol��ɫ����Y��Y�����������ֺ���ɫ��
��Ũ������Ũ�����Ϲ����л�������������嵥��X��������M����ɫҺ�壬������M������ԭ�Ӿ��ﵽ8���ӵ��ȶ��ṹ��2molM���ȷֽ�����lmol X��2mol��ɫ����Y��Y�����������ֺ���ɫ��
 ��
�� �����ʼ��仯����������������й㷺Ӧ�ã���ش��������⣺
�����ʼ��仯����������������й㷺Ӧ�ã���ش��������⣺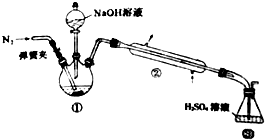 ijС���Է���м��ϡ���ᡢ���ͣ�NH4��2 SO4��ҺΪԭ�ϣ�����һϵ�з�Ӧ�Ͳ����ϳ���dz����ɫ����X��Ϊȷ������ɣ���������ʵ�飺
ijС���Է���м��ϡ���ᡢ���ͣ�NH4��2 SO4��ҺΪԭ�ϣ�����һϵ�з�Ӧ�Ͳ����ϳ���dz����ɫ����X��Ϊȷ������ɣ���������ʵ�飺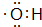 ��
�� ��ϵͳ����Ϊ3-�����飮
��ϵͳ����Ϊ3-�����飮 ��
��