��Ŀ����
��8�֣�ǰ���ñ������ҹ���ú���͡������ѽ��������Ƚ����У���ѧ��ͨ����иŬ�����ҵ��� ����ú��ˮ���������Ƶã�Ӧ�õ��·������磺��
����ú��ˮ���������Ƶã�Ӧ�õ��·������磺��
�������ϳ����ͣ� ��
�� ��443��473K���¶����������������ɺϳ�̼ԭ����Ϊ5��8��������
��443��473K���¶����������������ɺϳ�̼ԭ����Ϊ5��8��������
��д�� CO�ϳ����� �Ļ�ѧ����ʽ
�Ļ�ѧ����ʽ
�������ܱյĺϳ�����ͨ��ǡ������ȫ��Ӧ��CO�� ������ȫ��Ӧʱ����ѹ����ԭ����2/5�����¶Ȳ��䣩����ʱ ����С���û�С����������ɣ�������
������ȫ��Ӧʱ����ѹ����ԭ����2/5�����¶Ȳ��䣩����ʱ ����С���û�С����������ɣ�������
��Ҫ�ﵽ�����ϳ����͵�Ҫ��CO�� ������ȵ�ȡֵ��Χ��
������ȵ�ȡֵ��Χ��
(2)�ϳɼ״�����390��C��3.03��105Paʱ�� ����n��ʾ
����n��ʾ ��
�� �����ʵ���֮�ȣ�a��ʾ
�����ʵ���֮�ȣ�a��ʾ ��ת���ʣ�x��ʾ�ﵽ��Ӧ��ʱ�������
��ת���ʣ�x��ʾ�ﵽ��Ӧ��ʱ������� �������������
������������� ��a��x�Ĺ�ϵʽΪ ��
��a��x�Ĺ�ϵʽΪ ��
 ����ú��ˮ���������Ƶã�Ӧ�õ��·������磺��
����ú��ˮ���������Ƶã�Ӧ�õ��·������磺���������ϳ����ͣ�
 ��
�� ��443��473K���¶����������������ɺϳ�̼ԭ����Ϊ5��8��������
��443��473K���¶����������������ɺϳ�̼ԭ����Ϊ5��8����������д�� CO�ϳ�����
 �Ļ�ѧ����ʽ
�Ļ�ѧ����ʽ�������ܱյĺϳ�����ͨ��ǡ������ȫ��Ӧ��CO��
 ������ȫ��Ӧʱ����ѹ����ԭ����2/5�����¶Ȳ��䣩����ʱ ����С���û�С����������ɣ�������
������ȫ��Ӧʱ����ѹ����ԭ����2/5�����¶Ȳ��䣩����ʱ ����С���û�С����������ɣ������� ��Ҫ�ﵽ�����ϳ����͵�Ҫ��CO��
 ������ȵ�ȡֵ��Χ��
������ȵ�ȡֵ��Χ�� (2)�ϳɼ״�����390��C��3.03��105Paʱ��
 ����n��ʾ
����n��ʾ ��
�� �����ʵ���֮�ȣ�a��ʾ
�����ʵ���֮�ȣ�a��ʾ ��ת���ʣ�x��ʾ�ﵽ��Ӧ��ʱ�������
��ת���ʣ�x��ʾ�ﵽ��Ӧ��ʱ������� �������������
������������� ��a��x�Ĺ�ϵʽΪ ��
��a��x�Ĺ�ϵʽΪ ����������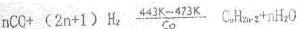 ��û��;��һ�������£�ѹǿ�ȵ������ʵ���֮�ȣ�= ��n=3���������͵�nֵ��5��11֮�䣬���˷�Ӧ��nֵΪ3���ʴ�ʱ���������ɡ���5/11��8/17(2) x=
��û��;��һ�������£�ѹǿ�ȵ������ʵ���֮�ȣ�= ��n=3���������͵�nֵ��5��11֮�䣬���˷�Ӧ��nֵΪ3���ʴ�ʱ���������ɡ���5/11��8/17(2) x=
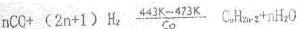 ��û��;��һ�������£�ѹǿ�ȵ������ʵ���֮�ȣ�= ��n=3���������͵�nֵ��5��11֮�䣬���˷�Ӧ��nֵΪ3���ʴ�ʱ���������ɡ���5/11��8/17(2) x=
��û��;��һ�������£�ѹǿ�ȵ������ʵ���֮�ȣ�= ��n=3���������͵�nֵ��5��11֮�䣬���˷�Ӧ��nֵΪ3���ʴ�ʱ���������ɡ���5/11��8/17(2) x=��������ϢΪ���壬�����˻�ѧ����ʽ����д����ѧƽ��ļ��㣻��1��CO��H2��һ�������¿ɺϳ���������Ӧ�Ļ�ѧ����ʽΪ��
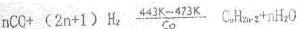 ����һ�������£�ѹǿ�ȵ������ʵ���֮�ȣ�= ��n=3���������͵�nֵ��5��11֮�䣬���˷�Ӧ��nֵΪ3���ʴ�ʱ���������ɡ���Ҫ�������ͣ���n=5ʱ����CO��H2�������Ϊ5��11����n=8ʱ����CO��H2�������Ϊ8��17����2����CO�����ʵ���ΪA��H2�����ʵ���ΪB��
����һ�������£�ѹǿ�ȵ������ʵ���֮�ȣ�= ��n=3���������͵�nֵ��5��11֮�䣬���˷�Ӧ��nֵΪ3���ʴ�ʱ���������ɡ���Ҫ�������ͣ���n=5ʱ����CO��H2�������Ϊ5��11����n=8ʱ����CO��H2�������Ϊ8��17����2����CO�����ʵ���ΪA��H2�����ʵ���ΪB��
CO + 2H2 ����CH3OH
��ʼ��Amol Bmol 0
ת����Aa 2Aa Aa
ƽ�⣺A-Aa B-2Aa Aa
x= ��=n��B��nA,����ã�x=
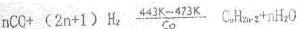 ����һ�������£�ѹǿ�ȵ������ʵ���֮�ȣ�= ��n=3���������͵�nֵ��5��11֮�䣬���˷�Ӧ��nֵΪ3���ʴ�ʱ���������ɡ���Ҫ�������ͣ���n=5ʱ����CO��H2�������Ϊ5��11����n=8ʱ����CO��H2�������Ϊ8��17����2����CO�����ʵ���ΪA��H2�����ʵ���ΪB��
����һ�������£�ѹǿ�ȵ������ʵ���֮�ȣ�= ��n=3���������͵�nֵ��5��11֮�䣬���˷�Ӧ��nֵΪ3���ʴ�ʱ���������ɡ���Ҫ�������ͣ���n=5ʱ����CO��H2�������Ϊ5��11����n=8ʱ����CO��H2�������Ϊ8��17����2����CO�����ʵ���ΪA��H2�����ʵ���ΪB��CO + 2H2 ����CH3OH
��ʼ��Amol Bmol 0
ת����Aa 2Aa Aa
ƽ�⣺A-Aa B-2Aa Aa
x= ��=n��B��nA,����ã�x=

��ϰ��ϵ�д�
�����Ŀ
 CO(g) + H2(g)��Ӧ��һ�������´ﵽƽ�⣬������ˮ����Ũ�ȣ�ƽ���� ��Ӧ�����ƶ�������������桱������ͬ����CO��Ũ�Ƚ� �������������С�����䡱��ͬ��
CO(g) + H2(g)��Ӧ��һ�������´ﵽƽ�⣬������ˮ����Ũ�ȣ�ƽ���� ��Ӧ�����ƶ�������������桱������ͬ����CO��Ũ�Ƚ� �������������С�����䡱��ͬ�� N2O4��g�� ������Ѹ��������ʱ��ƽ���� ��Ӧ�����ƶ����������ɫ�ȱ�_____����dz����������䡱��ͬ�������______�����պ������ȣ�_____����ǰ������
N2O4��g�� ������Ѹ��������ʱ��ƽ���� ��Ӧ�����ƶ����������ɫ�ȱ�_____����dz����������䡱��ͬ�������______�����պ������ȣ�_____����ǰ������ CO��g��+ H2O��g�� ��H =" Q" kJ��mol��1
CO��g��+ H2O��g�� ��H =" Q" kJ��mol��1 ����H2����
����H2���� 2SO3(g)
2SO3(g) 2SO3����H��0����ʱ�����ѱ��־�ֹ��SO2��Ӧ����Ϊv0������A��Ѹ�ٳ���2 mol SO2��1 mol O2���������ٴα��־�ֹʱ��SO2��Ӧ����Ϊv���ڴ˹����У�����˵����ȷ����
2SO3����H��0����ʱ�����ѱ��־�ֹ��SO2��Ӧ����Ϊv0������A��Ѹ�ٳ���2 mol SO2��1 mol O2���������ٴα��־�ֹʱ��SO2��Ӧ����Ϊv���ڴ˹����У�����˵����ȷ����

 XeF4(g)+F2(g),�䷴Ӧ�Ȧ�H0(�������������������������__________________________��
XeF4(g)+F2(g),�䷴Ӧ�Ȧ�H0(�������������������������__________________________�� 2SCl2(�ʺ�ɫҺ��)����H=+61.16kJ��
2SCl2(�ʺ�ɫҺ��)����H=+61.16kJ�� FeO(s) �� CO(g)�����¶���ƽ�ⳣ��K�Ĺ�ϵ���±���
FeO(s) �� CO(g)�����¶���ƽ�ⳣ��K�Ĺ�ϵ���±���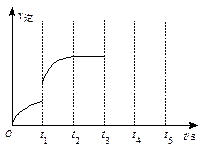
 ����t3~ t5ʱ��ε�v���仯���ߡ�
����t3~ t5ʱ��ε�v���仯���ߡ� 2AB��g���ﵽƽ��״̬�ı�־��( )
2AB��g���ﵽƽ��״̬�ı�־��( )