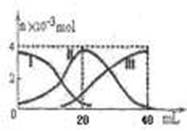
��Ŀ����
�����ʵ��Ļ�ѧ������գ�
��1��Na2CO3ˮ������ӷ���ʽ��______��
��2��H2S���뷽��ʽ��______��
��3��AlCl3ˮ������ӷ���ʽ��______��
��4����25�桢101kPa�£�lg������ȫȼ������CO2��Һ̬ˮʱ����55.6kJ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��______��
��5����������ȼ�ϵ�ص������缫����ʽ
������______��������______��
��6��д��NaHCO3��Һ�е�����Ũ�ȹ�ϵ
c��H+��+c��Na+��=______��
c��Na+��=______��
��1��Na2CO3ˮ������ӷ���ʽ��______��
��2��H2S���뷽��ʽ��______��
��3��AlCl3ˮ������ӷ���ʽ��______��
��4����25�桢101kPa�£�lg������ȫȼ������CO2��Һ̬ˮʱ����55.6kJ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��______��
��5����������ȼ�ϵ�ص������缫����ʽ
������______��������______��
��6��д��NaHCO3��Һ�е�����Ũ�ȹ�ϵ
c��H+��+c��Na+��=______��
c��Na+��=______��
��1��̼����ˮ��Һ��ˮ�⣬̼��������Ƕ�Ԫ���������ӷֲ�ˮ�⣬ˮ������ӷ���ʽΪ��CO32-+H2O?OH-+HCO3-���ʴ�Ϊ��CO32-+H2O?OH-+HCO3-��
��2��������������ʣ���ˮ��Һ�д��ڵ���ƽ�⣬��Ԫ����ֲ����룬����ķ���ʽΪ��H2S?H++HS-���ʴ�Ϊ��H2S?H++HS-��
��3���Ȼ���ˮ��Һ��������ˮ�����������������Ȼ��⣬�ʴ�Ϊ��Al3++3H2O?Al��OH��3��+3H+��
��4��lg������ȫȼ������CO2��Һ̬ˮʱ����55.6kJ������16g����ȼ�շ���889.6KJ�������Ȼ�ѧ����ʽ��д������ע�ۼ�״̬�Ͷ�Ӧ�ʱ�д���Ȼ�ѧ����ʽΪ��
CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-889.6 kJ/mol��
�ʴ�Ϊ��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-889.6kJ/mol��
��5������ȼ�ϵ��ԭ�������������ڸ���ʧ���ӷ���������Ӧ��������Һ������ˮ�������缫��ӦΪ��2H2-4e-+4OH-=4H2O�������õ������������������ӣ������缫��ӦΪ��O2+4e-+2H2O=4OH-��
�ʴ�Ϊ��2H2-4e-+4OH-=4H2O��O2+4e-+2H2O=4OH-��
��6��̼��������Һ�д��ڵ���غ�Ϊ��c��Na+��+c��H+��=c��OH-��+2c��CO32-��+c��HCO3-���������غ�����Ԫ�غ�̼Ԫ�ص��غ㣬���ݻ�ѧʽ��֪��Ԫ�غ�̼Ԫ����ͬ��̼Ԫ�صĴ�����ʽ��̼���������ˮ��͵��뷢���ı䣬�õ������غ�Ϊc��Na+��=c��HCO3-��+c��CO32-��+c��H2CO3����
�ʴ�Ϊ��c��OH-��+2c��CO32-��+c��HCO3-����c��HCO3-��+c��CO32-��+c��H2CO3����
��2��������������ʣ���ˮ��Һ�д��ڵ���ƽ�⣬��Ԫ����ֲ����룬����ķ���ʽΪ��H2S?H++HS-���ʴ�Ϊ��H2S?H++HS-��
��3���Ȼ���ˮ��Һ��������ˮ�����������������Ȼ��⣬�ʴ�Ϊ��Al3++3H2O?Al��OH��3��+3H+��
��4��lg������ȫȼ������CO2��Һ̬ˮʱ����55.6kJ������16g����ȼ�շ���889.6KJ�������Ȼ�ѧ����ʽ��д������ע�ۼ�״̬�Ͷ�Ӧ�ʱ�д���Ȼ�ѧ����ʽΪ��
CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-889.6 kJ/mol��
�ʴ�Ϊ��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-889.6kJ/mol��
��5������ȼ�ϵ��ԭ�������������ڸ���ʧ���ӷ���������Ӧ��������Һ������ˮ�������缫��ӦΪ��2H2-4e-+4OH-=4H2O�������õ������������������ӣ������缫��ӦΪ��O2+4e-+2H2O=4OH-��
�ʴ�Ϊ��2H2-4e-+4OH-=4H2O��O2+4e-+2H2O=4OH-��
��6��̼��������Һ�д��ڵ���غ�Ϊ��c��Na+��+c��H+��=c��OH-��+2c��CO32-��+c��HCO3-���������غ�����Ԫ�غ�̼Ԫ�ص��غ㣬���ݻ�ѧʽ��֪��Ԫ�غ�̼Ԫ����ͬ��̼Ԫ�صĴ�����ʽ��̼���������ˮ��͵��뷢���ı䣬�õ������غ�Ϊc��Na+��=c��HCO3-��+c��CO32-��+c��H2CO3����
�ʴ�Ϊ��c��OH-��+2c��CO32-��+c��HCO3-����c��HCO3-��+c��CO32-��+c��H2CO3����

��ϰ��ϵ�д�
 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�
�����Ŀ
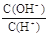 =10��12����Һ�У� K+��ClO����S2���� Cl��
=10��12����Һ�У� K+��ClO����S2���� Cl��