��Ŀ����
�����£����и���������ָ����Һ�п��ܴ����������:
| A����������Ժ�ɫ����Һ�У� Na����NO3���� Fe2+��SO32�� |
| B��ˮ������� c ( H+) = 10��12 mol/L ����Һ��:K+�� AlO2���� CH3COO���� Cl�� |
C��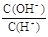 =10��12����Һ�У� K+��ClO����S2���� Cl�� =10��12����Һ�У� K+��ClO����S2���� Cl�� |
| D��c ( Fe 3+ ) =" 0" .1 mol/L ����Һ�У� K+��Cl�� ��SO42���� SCN�� |
B
���������A����������Ժ�ɫ����ҺΪ���ԣ� NO3����Fe2+��SO32�����������棻B��ˮ�������c(H+)=10��12 mol/L����Һ����Ϊ���ԣ�AlO2����CH3COO�����ܴ������棬����Ϊ���ԣ����Դ������棻C��
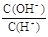 =10��12��Һ��c(H+)=10-1mol/L����������Һ��ClO����S2������������ԭ��Ӧ��D��c(Fe3+)="0.1" mol/L ����Һ�в�����SCN����Fe3+���䷢����Ϸ�Ӧ��
=10��12��Һ��c(H+)=10-1mol/L����������Һ��ClO����S2������������ԭ��Ӧ��D��c(Fe3+)="0.1" mol/L ����Һ�в�����SCN����Fe3+���䷢����Ϸ�Ӧ��
��ϰ��ϵ�д�
�����Ŀ